Introduction
Daily laboratory activity is an essential part of the comprehensive patient care, and consists of various actions that should be optimized and standardized to provide laboratory reports that might be ultimately useful for the clinical decision making (1). As the number and complexity of laboratory analyses are constantly increasing, it is essential that laboratory errors are reduced to the least possible rate. It has been estimated that 0.1 to 9.3% of all laboratory reports might contain errors (2,3). Laboratory diagnostics is traditionally divided into three phases, which are pre-analytical, analytical and post-analytical. Development of technology, automatization and quality control of analytical processes has contributed to reduce significantly the rate of errors in the analytical phase (4,5). Nevertheless, potential errors still can arise from the extra-analytical phase (3-9). It is estimated that out of all errors in laboratory daily activity, 13% come from analytical, 46-68% from pre-analytical and 18-47% from post-analytical procedures (3,9), so that major focus should be placed to improve extra-analytical phase quality. Since the problem of diagnostic errors overcomes laboratory area, the International Organization for Standardization (ISO) has proposed a broader definition of the term “laboratory error”, which is now referred to as any “nonconformity” which can occur in every process of the total testing process, from test request to analysis reporting, result interpretation and medical actions undertaken according to test results (10).
According to the Croatian Law, laboratory routine activity has to be supervised and controlled by medical biochemists having at least master’s degree and who have passed state exam and received license for individual activity as issued by the Croatian Chamber of Medical Biochemists (CCMB). Further professional qualification is gained through specialization which gives credibility for higher ranking responsibilities in governing and organizing laboratory activity.
Medical biochemist is obliged to control all phases of daily laboratory routine, in particular those concerning internal quality control (IQC), and to participate in mandatory program of external quality assessment (EQA) of the analytical phase, which is run and supervised by the Croatian Society of Medical Biochemists (CSMB). The EQA of extra-analytical phases is however not mandatory, as in some other countries. In order to follow new trends of good laboratory practice, an initiative for pre-analytical and post-analytical quality control implementation has been designed at national level. The process of accreditation in health care is also being prepared at national level and has become an important part of healthcare development policy in Croatia. Accreditation according to international standards is still neither obligatory nor defined by Croatian law for medical laboratories. There are however guidelines and recommendations for standardization issued by the Croatian Chamber of Medical Biochemists (CCMB). A variety of laboratories in Croatia have a plan to start accreditation process in a short time. As such, it becomes essential to identify critical points in laboratory activity and to systematically monitor all laboratory processes aimed to raise the global quality and to prepare for administratively demanding process of accreditation.
A cross-sectional survey study was therefore performed among members of CCMB, with the aim to investigate the status of the extra-analytical phase in Croatia. The leading objective was to detect and target the most critical points of the extra-analytical phase. The study was based on self-reported evaluation on frequency of some procedures concerning the extra-analytical phase, and was administered to personnel responsible of laboratory activity. The main results are aimed to identify those procedures needing further improvement and rapid intervention. An additional aim was to identify potential associations between self reported extra-analytical quality of laboratory activity and type of facility, degree of professional qualification and informatics skills of those responsible of laboratory management.
Materials and methods
Questionnaire
An anonymous questionnaire, consisting of 20 closed questions describing procedures of extra-analytical phase of laboratory diagnostics, was sent by regular mail to all members of CCMB in April 2009. Participants were identified using the database of the CCMB. Data on type of the laboratory (e.g., institution), age, gender, professional qualification and informatics skills were also collected.
The questions were designed as statements describing laboratory procedures and answer were offered as frequency of particular procedure performed in participant’s laboratory on a four grade Likert scale, graded as 1 (never), 2 (rarely), 3 (often) and 4 (always). Negative statements (5 questions) were recoded (graded 4 for “never” to 1 for “always” respectively). As such, higher score represents higher quality of extra-analytical phase laboratory procedures. The reliability of the questionnaire was determined by Cronbach’s alpha coefficient (α = 0.75), which is globally acceptable. Results were calculated as an average grade (e.g. score for each participant), and compared between groups.
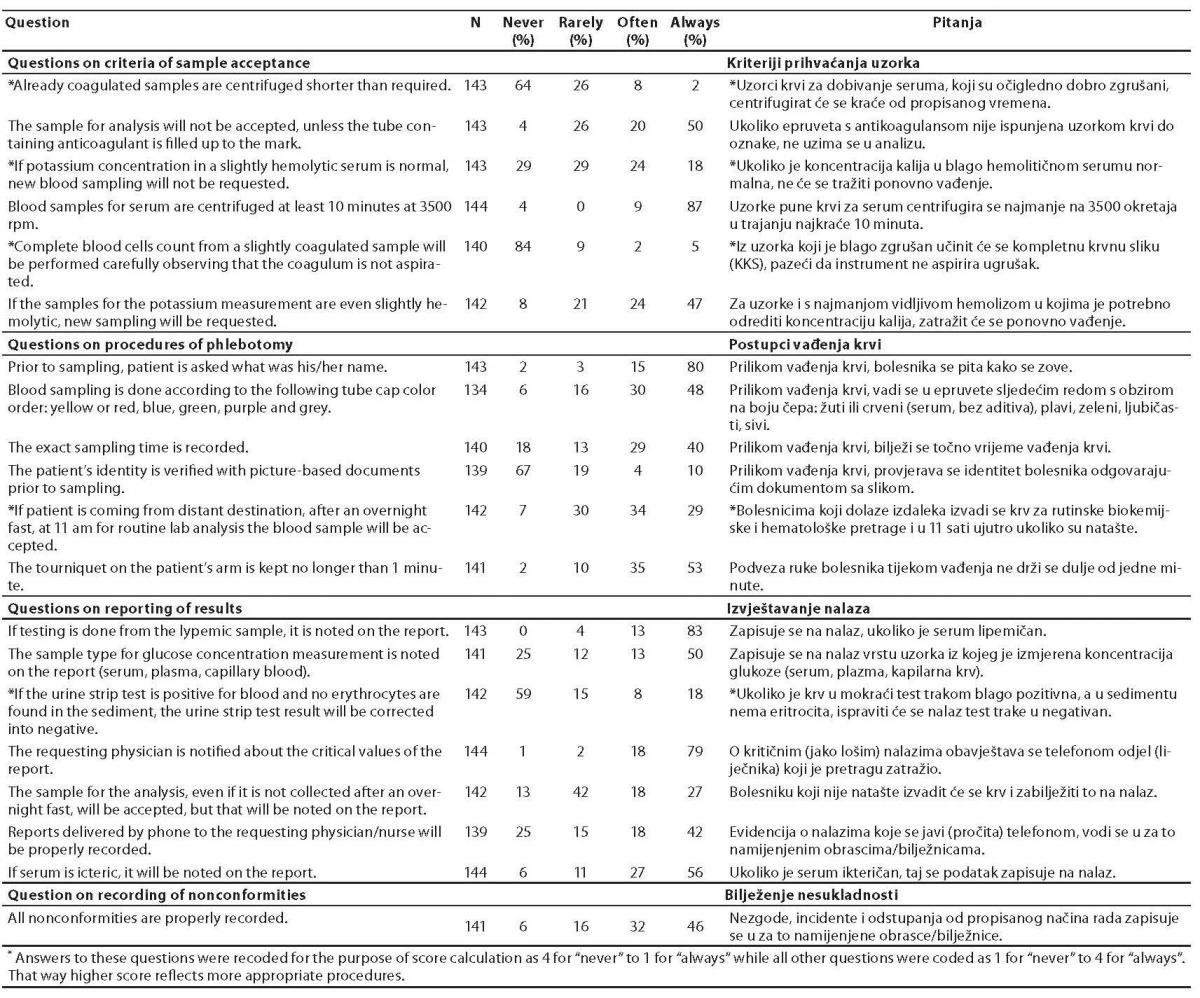
Along with the overall score concerning all questions, three more scores were calculated. The questions were also divided in three groups, including questions considering criteria of sample acceptance (6 items), questions considering procedures of phlebotomy (6 items) and questions considering reporting of results (7 items). The corresponding scores were calculated respectively. The question regarding the practice of recording nonconformities was evaluated separately (Table 1).
Table 1. Distribution of answers regarding occurrence of procedures of extra-analytical phase of laboratory diagnostics among medical biochemists in Croatia.
Statistical analysis
Results are presented as numbers and percentages for frequencies and as mean and standard deviation for scores common for data concerning grades. The difference in average score between two groups was calculated using independent sample t-test and ANOVA between three or more groups, followed by Student-Newman-Keuls post-hoc test to find out differences in between groups. Correlation between age and average score were assessed by calculating Pearson's correlation coefficient. P value less than 0.05 was consider significant for all statistical tests.
All statistical analyses were performed using MedCalc statistical software version 10.0.1.0. (MedCalc, Mariakerke, Belgium) licensed to Department of Medical Informatics, Rijeka University School of Medicine.
Results
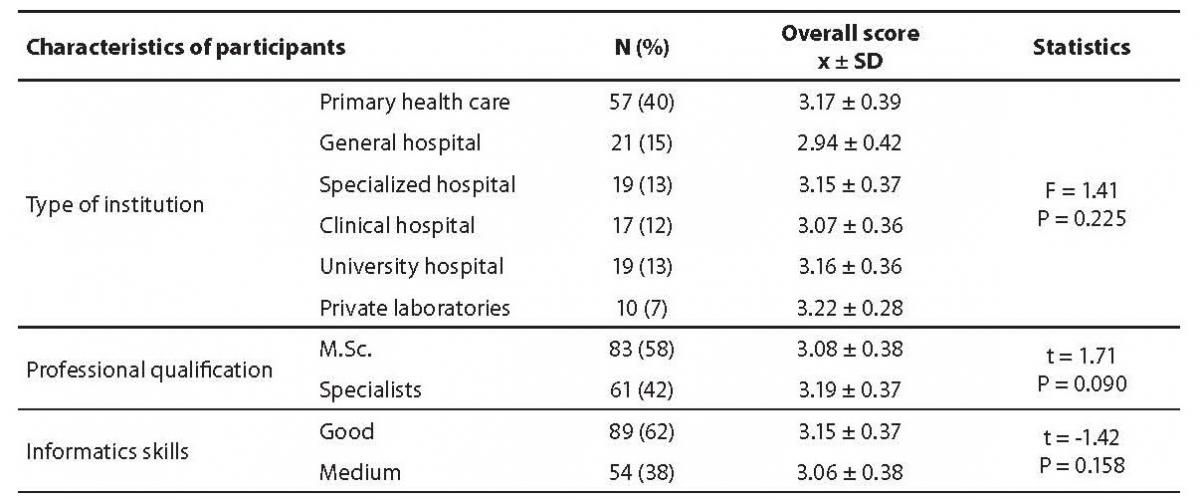
Out of the 538 members of CCMB, 144 (27%) completed the questionnaire; 93% of them were women; the median age was 47 years and ranged from 26 to 65 years. There were 58% medical biochemists with master degree, and 42% specialist of medical biochemistry. Out of all subjects, 40% worked in primary care laboratories, 15% in laboratories of general hospital, 13% in laboratories of specialized hospital (e.g. psychiatric, rehabilitation, cardio-surgical hospital, etc), 12% of clinical hospital's laboratories, 13% in laboratories of university hospital and 7% in private laboratories. As regards the informatics skills, 38% of participants self-reported medium and 62% good skills.
The distribution of the answers to the questions is shown in table 1. The overall score was calculated according to four grade Likert scale after recoding of negative statements (table 1, marked with asterisk) and mean ± standard deviation (SD) of overall score was 3.12 ± 0.38. There was no statistically significant difference between groups considering type of facility (P = 0.225), professional qualification (P = 0.090) or informatics skills (P = 0.158) (Table 2). There was also no correlation between age and overall score (r = 0.21, P = 0.011).
Table 2. Comparison of overall score obtained through questionnaire regarding participants from different type of institution, professional qualification and their informatics skills.
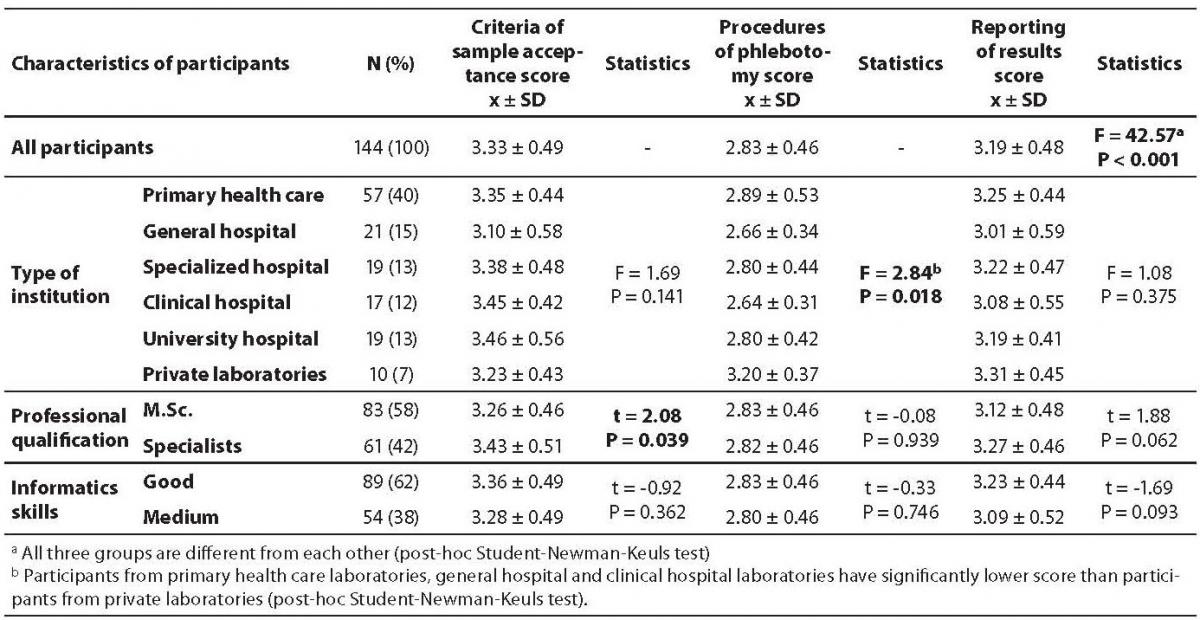
As questions were divided in three groups, the score for each group were calculated as follows (mean ± SD): 3.33 ± 0.49 for questions on criteria of sample acceptance; 2.83 ± 0.46 for questions on procedures of phlebotomy and 3.19 ± 0.48 for questions on results reporting. All three groups significantly differ from each other (P < 0.001), the lowest score being recorded for phlebotomy procedures (Table 3).
According to type of facility, no difference was observed in the score for questions regarding criteria of sample acceptance and results reporting, though a significant difference for questions regarding procedures of phlebotomy was observed (P = 0.018), where participants from primary health care laboratories (2.89 ± 0.53), general hospital (2.66 ± 0.34) and clinical hospital laboratories (2.64 ± 0.31) had significantly lower scores than participants from private laboratories (3.20 ± 0.37) (Table 3).
Table 3. Comparison of criteria of sample acceptance score, procedures of phlebotomy score and reporting of results score obtained through questionnaire between each other and between participants from different type of institution, professional qualification and their informatics skills.
Criteria of sample acceptance score was significantly lower in group of medical biochemists with master degree than in those being specialists (3.26 ± 0.46 vs. 3.43 ± 0.51; P = 0.039) (Table 3).
No difference in any of the scores regarding information skills was observed. As regards the question concerning the procedure of recording nonconformities, 22% of participants did not record nonconformities properly (e.g., never or rarely), whereas 78% of participants recorded nonconformities often or always (Table 1).
Discussion
The overall score of 3.12 ± 0.38 on a scale from 1 to 4 can be considered a reliable indicator of medium quality standards in our survey. The lack of difference in the overall score among the different groups also highlighted that type of laboratory, professional qualification and informatics skills of medical biochemists responsible for laboratory activity are not directly associated with the quality of the extra-analytical phase of laboratory practice. However, when questions were further classified in three groups, some difference emerged. The first and the most indicative finding is that questions on procedures of phlebotomy achieved the lowest score (2.83 ± 0.46); an intermediate score was observed for results reporting (3.19 ± 0.48), whereas the highest score was recorded for criteria of sample acceptance (3.33 ± 0.49), all these significantly differing the one from another.
The phlebotomy practice is a well known and recognized problem in published studies, as probably the most critical issue in extra-analytical phase (11-14). Despite significant improvements during last two decades in blood sampling equipments and procedures (positive patient identification, vacuum tubes for blood sampling, more convenient needles for phlebotomy etc.), it is still important to emphasize that procedures of phlebotomy need a further attention and a more careful and systematic supervision in order to prevent errors. Importantly, laboratory personnel might probably make fewer mistakes during phlebotomy than other non-laboratory personnel performing it (e.g. nurses, physicians) (15), so that it is essential to extend supervision and continuous education outside the walls of the laboratory, to all the personnel involved in this activity (12,13,15).
Sixty three percent of biochemists report that blood samples are accepted in their laboratories from patients coming from distant destinations, after overnight fasting at 11 AM for routine analyses (table 1). To accomplish the patients, saving them another visit to hospital laboratory, personnel often contravene rules of good laboratory practice and take blood sample in improper time and causing possible erroneous results that might impact on both, quality and patient’s health. Compassionate reasons for blood sampling from patients coming too late, fasting too long or not fasting at all are obviously not in patient’s favor.
Although 80% of participants self-reported to ask patient’s name prior to blood sampling, only 14% declared that patient identification is often or always verified with official (picture based) document (Table 1). The procedure of identity verification is often not regulated by guidelines of laboratory practice, but it still can be a serious source of errors. There are several studies published with discussion of patient’s identity verification issue. Misidentification of patients is acknowledged as a major cause of medical errors (12,16,17). In several developed countries there is a well established procedure for patient identification, for example wearing bar-coded wristbands (16) in USA hospitals, or checking and recording national identification number in Sweden (15). Bar-coded wrist bands significantly reduced misidentification in hospitals, but problem of identification in high turnover phlebotomy services is still a central issue regarding medical errors (12,16,17). As such, it seems of pivotal importance to have reliable system of identity verification, to prevent incidental mismatch or even abusive attempt of switching identity.
According to our results, one third of the laboratories still do not pay attention on sampling time recording. As such, it would be impossible to check if a proper time to analyses has been respected, and serious errors can consequently occur for those analytes which concentration might significantly change even over shorter period of time (e.g., electrolytes, lactate, glucose, etc.). We thereby suggest that the time of sampling should be used as the starting point for recording the TAT (turn-around time), inasmuch as TAT is one of the important quality control indicators and it might be so impossible to introduce proper quality management when TAT is not appropriately recorded (16,18).
The score recorded for phlebotomy procedures is not apparently associated with professional qualification or informatics skills of biochemists, but it is instead with the type of institution. The highest score regarding procedures of phlebotomy is obtained from participants working in private laboratories (3.20 ± 0.37), whereas all others obtained scores less than 3.0 (Table 3). Participants from private laboratories (7% of all participants) were obviously paying more attention to blood sampling than others, while scores for other procedures such as criteria for sample acceptance and results reporting were not significantly different between different types of facility (table 3). Private laboratories inherently work in open market, in strong competition, so that it can be assumed that they might be much more sensitive to blood sampling issues, where stakeholders (e.g., patients) are involved ensuring the highest possible comfort for patients and avoidance of any unnecessary sampling repetition.
Result reporting is considered to be the leading part of the post-analytical phase and despite of higher score than those of phlebotomy procedures it can and has to be further improved. Although almost all participants reported that they would promptly inform the requesting physician on critical values, 40% of them will never or rarely record that they reported result by phone. Such behavior prevents the tracing of the reports, and introduces a strong possibility that erroneous results can be written down and the impossibility to prove results reporting when requested or necessary. It is of outmost importance to record verbally reported results, and to be sure that the appropriate person receives and correctly notes information on critical result (16). Unresolved phone calls happen to be the second most frequent error in extra-analytical phase (19). Traditionally, actions which are not recorded might be actually claimed as not happened (20).
Correction of results for blood in urine obtained by urine strip test according to microscopic finding of erythrocytes in the urinary sediment is still performed by one quarter of participants. This procedure has no real justification due to possible presence of free hemoglobin or myoglobin in urine regardless of the presence of erythrocytes that is a clinically important finding.
Questions on criteria of sample acceptance achieved the highest score and the vast majority of participants handled this part of the pre-analytical phase satisfactory. Interestingly, the score for sample acceptance criteria was significantly higher in a group of biochemists with specialization, rather than in those with master’s degree (3.43 ± 0.51 vs. 3.26 ± 0.49; P = 0.039; table 3). It can hence be conceived that a higher level of professional qualification leads to major awareness of the importance of the extra-analytical phases, and there is also a possibility that they are involved in preprocesses for accreditation which includes strict supervision of all laboratory phases.
Our assumption that higher informatics skills will be associated with quality of extra-analytical procedures could not be confirmed. The majority of participants (62%) self-reported good informatics skills, and no participants reported poor skills. The age of participants was also not associated with the score, thus confuting the hypothesis that elderly participants might be more permissive to rules of extra-analytical phase than the younger ones.
The essential basis and mandatory request of quality control system of all phases, especially the extra-analytical ones, is the appropriate recording of all nonconformities. Still 6% of all participants never record nonconformities, 16% do that just rarely and half of them (46%, table 1) do it always. This revealed that half of participants are probably not aware of mistakes that regularly happen in their laboratories. All nonconformities have to be properly recorded in all cases to have a strict control of the processes, to recognize errors and to develop quality system and appropriate actions for correction and prevention of recurrences of similar events (3,20,21).
A major limitation in this study is the modest response rate (27%). Although we assume that the sample is still representative, it is possible that only participants with high interest and motivated have answered to the questionnaire, whereas those who did not find the study of importance did not, so that results might have been partially biased.
The other limitation is the possibility of socially desirable answers. As such, some participants might have reported desirable rather than true answers regardless of the anonymity of the survey (as previously highlighted results and conclusions are only based on self-reported data). An additional limitation is the fact that responders often do not perform some or even all of the described procedures themselves, but the laboratory technicians do so, the biochemists being only responsible for a global supervision of the activity. The questionnaire was completely designed by authors thus reflecting their attitudes and work experience in several different laboratories in Croatia. Considering that the importance of some items (e. g. described procedures) as quality indicators can be under or over estimated that can be subject of further discussion.
We can thereby conclude that results of quality of the extra-analytical phase of laboratory diagnostics in Croatia are satisfactory, but we also highlight the urgent need for further improvement, as in other countries. If high quality standards are to be reached, it is mandatory to supervise and record properly all laboratory procedures. If all procedures and nonconformities are not appropriately flowed and recorded, the laboratory management might not be aware of errors that can occur. With the clear perception that the vast majority of laboratory errors arise from the extra-analytical phase, further education of all the personnel involved, along with introduction of proper identification, recording, control and management of errors in the extra-analytical phase will improve laboratory process and improve the quality of the total testing process. In this period, anticipating certification and accreditation, it is mandatory that all possible sources of errors come to the spotlight and that procedure for their recognizing, prevention and constant control are introduced.
Acknowledgments
Authors are thankful to the Croatian Chamber of Medical Biochemists for providing contacts to members and for technical and finance help in sending and collecting surveys by regular mail. Authors are especially grateful to all members who fulfilled the questionnaire realizing the importance of quality improvement and thus made this study possible.
Notes
Potential conflict of interest
None declared.
References
1. Regan M, Forsman R. The impact of the laboratory on disease management. Dis Manag 2006;9:122-30.
2. Bonini PA, Plebani M, Ceriotti F, Ruboli FF. Errors in laboratory medicine. Clin Chem 2002;48:691-8.
3. Kalra J. Medical errors: impact on clinical laboratories and other critical areas. Clin Biochem 2004;37:1052-62.
4. Plebani M. Exploring the iceberg of errors in laboratory medicine. Clin Chem Acta 2009;404:16-23.
5. Plebani M, Carraro P. Mistakes in a stat laboratory: types and frequency. Clin Chem 1997;43:1348-51.
6. Plebani M. Laboratory errors: How to improve pre- and post-analytical phases? Biochem Med 2007;17:5-9.
7. Carraro P, Plebani M. Errors in stat laboratory: types and frequencies 10 years later. Clin Chem 2007;53:1338-42.
8. Lippi G, Guidi GC, Mattiuzzi C, Plebani M. Preanalytical variability: the dark side of the moon in laboratory testing. Clin Chem Lab Med 2006;44:358-65.
9. Lippi G. Governance of preanalytical variability: Travelling the right pith to the bright side of the moon? Clin Chim Acta 2009;404:32-6.
10. International Organization for Standardization. ISO/PDTS 22367. Medical laboratories: reducing error through risk management and continual improvement:complementary element. ISO, Geneva, 2005:9.
11. Wallin O, Söderberg J, Van Guelpen B, Stenlund H, Grankvist K, Brulin C. Preanalytical venous blood sampling practices demand improvement – A survey of test-request management, test-tube labelling and information search procedures. Clin Chim Acta 2008;391:91-7.
12. Lippi G, Salvagno GL, Montagnana M, Franchini M, Guidi GC. Phlebotomy issues and quality improvement in results of laboratory testing. Clin Lab 2006;52:217-30.
13. Söderberg J, Brulin C, Grankvist K, Wallin O. Preanalytical errors in primary healthcare: a questionnaire study of information search procedures, test request management and test tube labelling. Clin Chem Lab Med 2009;47:195-201.
14. Lippi G, Salvagno GL, Montagnana M, Guidi GC. The Skilled phlebotomist. Arch Pathol Lab Med 2006;130:1260-1.
15. Wallin O, Söderberg J, Van Guelpen B, Brulin C, Grankvist A. Patient-centred care – preanalitycal factors demand attention: A questionnaire study of venous blood sampling and specimen handling. Scand J Clin Lab Invest 2007;67:836-47.
16. Howanitz PJ. Errors in laboratory medicine: practical lessons to improve patient safety. Arch Pathol Lab Med 2005;129:1252-61.
17. Lippi G, Blanckaert N, Bonini P, Green S, Kitchen S, Palicka V, et al. Causes, consequences, detection, and prevention of identification errors in laboratory diagnostics. Clin Chem Lab Med 2009;47:143-53.
18. Simundic AM, Topic E. Quality indicators. Biochem Med 2008;18: 311-9.
19. Ricos C, Garcia-Victoria M, de la Fuente B. Quality indicators and specifications for extra-analytical phases in clinical laboratory management. Clin Chem Lab Med 2004;42:578-82.
20. Sciacovelli L, Plebani M. The IFCC working group on laboratory errors and patient safety. Clin Chim Acta 2009;404:79-85.
21. Schultz IJ, Kiemeney LA, Willems JL, Swinkels DW, Witjes JA, de Kik JB. Preanalytic Error Tracking in a Laboratory Medicine Department: Results of 1-Year Experience. Clin Chem 2006;52:1442-3.