Introduction
Diabetes mellitus is a chronic, progressive disease characterized by absolute or relative deficiency of the insulin hormone. Chronic hyperglycemia, which occurs due to insulin deficiency, causes the incidence of subsequent microvascular diabetic complications: retinopathy, neuropathy, and nephropathy. Diabetes is also an independent risk factor for the development of macrovascular, primarily cerebrovascular diseases (1). The most recent indicators point to a high prevalence of diabetes in Croatia (8.9%), with a high proportion (42%) of undiagnosed diabetes in adult population (2). Complex pathology, increased diabetes-related morbidity and mortality, and a growing number of patients are a considerable burden to the healthcare system, both at global and national level. Total healthcare costs for diabetic patients were estimated to be twofold those for non-diabetic individuals (3). On the other hand, severe disability and loss of work ability occurring due to late complications in diabetes exert a negative effect on the quality of life and social welfare. Hence any means to improve glycemia control and retard or prevent the incidence of late complications is valuable, as these are the goals of continuous development of therapeutic potentials. Laboratory medicine has also recognized the importance of its role in this area so that a program entitled “Global IFCC Drive on Diabetes” has been launched by the International Federation of Clinical Chemistry (IFCC) in order to integrate a series of activities related to laboratory diagnosis and follow-up of diabetes mellitus (4).
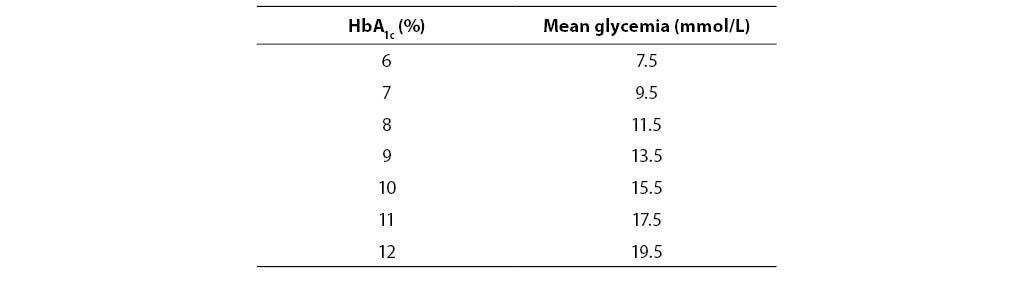
It is, however, interesting that therapeutic goals of this complex disease are based on results of a simple laboratory test, i.e. hemoglobin A1c (HbA1c) (5). Actually, results of long-term interventional studies DCCT (Diabetes Control and Complications Trial) and UKPDS (United Kingdom Prospective Diabetes Study) confirmed undeniable correlation between glycemic levels, HbA1c, and progression of late complications in diabetes, thus establishing the foundation for modern, globally accepted therapy guidelines and recommendations where the level of HbA1c £ 7% is defined as the target of good glycemia regulation (6–9).
Table 1. Correlation between HbA1c and mean glycemia
It is even more interesting that this generally accepted "gold standard", used to assess both therapy efficiency and the risk for occurrence of late diabetic complications, has not been analytically standardized after almost 30 years of use (10, 11). Interpretation of HbA1c results is further limited by inadequately recognized biological and clinical variations (12). All the above represents a considerable and often underestimated problem occurring during comparison of individual results with widely accepted professional guidelines and recommendations which imply the possibility of definite interpretation (13).
Results of our investigations of HbA1c analysis and clinical application in Croatia pointed to variability in analytical methodology, and poor availability and clinical utilization of the test which could be significantly improved by HbA1c inclusion among general medical biochemistry tests (14, 15). However, without awareness of all aspects of this controversial test issue and definition of clear quality criteria through regular implementation of professional surveillance, broad application of this test could be more harmful than beneficial for both diabetic patients and healthcare system. Hence the implementation of education at all levels and introduction of an extermal quality control program are at the moment an undeferrable necessity, while good knowledge, keeping up-to-date, and application of internationally accepted standards is the only way to harmonize HbA1c determination in Croatia.
Background
Erythrocytes of a healthy individual contain 90% of adult hemoglobin (HbA) while the remaining percentage are the products of alternative globin synthesis ((HbA2, HbF) and post-translational HbA modifications. As early as in 1958, three lesser hemoglobin components with the negative charge stronger than that of hemoglobin A were separated by cationic exchange chromatography (16). Based on elution sequence from cationic exchanger, the fractions were termed HbA1a, HbA1b i HbA1c. This discovery was related to diabetes several years later when an increase in concentration of “rapid” hemoglobin fractions was described in blood of diabetic patients (17). Further investigations revealed that HbA1c is a direct product of post-translational glucose binding to hemoglobin molecules, and that there is a correlation between HbA1c level and mean glucose concentration in blood during previous three months, which is the average life-time of erythrocytes (18, 19). The intriguing possibility to gain objective insight into mean glycemia during a prolonged period by a single measurment opened up a new dimension of diabetes control and follow-up. Concurrent development of analytical methodology soon enabled wide application of HbA1c in laboratory and clinical medicine.
Chemistry, technology and definitions
HbA1c and glycohemoglobin (GHb) are two distinct entities produced by glycation, i.e. the process of non-enzymic covalent glucose binding to free amino groups of globin chains (20). All proteins are subject to the glycation process which should be differentiated from glycosylation that represents an enzymic phase in the synthesis of membrane and other glycoproteins. On this account the term “glycosylated hemoglobin”, which was long used in professional literature, has been abandoned. The initial step in glycation is condensation of free primary amino acid of the protein chain with carbonyl glucose group, with an unstable byproduct, Schiff’s base. Depending on glucose concentration, it may dissociate or remodel into stable ketoamine form (21). N-terminal valine groups on a- and b-chains undergo glycation on the hemoglobin molecule, as well as e-amino lysin groups of a- and b-chains. The amount of produced GHb is directly proportional to the glucose concentration to which erythrocytes are exposed during circulation.
HbA1c is hemoglobin with the glucose bound to N-terminal valine groups on one or both b-globin chains, while total GHb represents hemoglobin molecules with covalently bound glucose in all above mentioned amino groups of a- and b-chains. Although HbA1c is only one of the components of total GHb (it contains approximately 60% of the total bound glucose), it is presently used as a standard measure of meanglycemia, chiefly due to clinical guidelines and recommendations based on results of large-scale interventional studies that involved HbA1c determination (22).
Methodology
Current analytical methods are varied and numerous, based on differences in physico-chemical properties between HbA1c/GHb molecules and unmodified hemoglobin (11, 23). Determination methods may be classified into two fundamental groups. The first group of methods is founded on differences in charge, encompassing ion-exchange chromatography and electrophoresis. The second group are structurally specific methods: borate affinity chromatography and immunochemistry. Immunochemical methods and the methods based on differences in charge are used to measure HbA1c, while affinity methods are used to determine total GHb. Each of the methods has some advantages and drawbacks so that the choice depends on individual needs and laboratory possibilities. Fulfilment of clinical and analytical quality criteria should be taken into account during application of these methods.
Clinical requirements:
Low individual variability.
Precision that enables making clinical decisions at the level of absolute difference in hemoglobin A1c from 0.35 – 0.5%.
Clear, unambiguous interpretation of results for physicians and patients based on DCCT and UKPDS criteria.
Analytical requirements:
Precise, stable methodology for hemoglobin A1c determination.
Total coefficient of variation <3.0%.
Stable reference/recommended values that eligible for uniform interpretation independently of the applied methodology, time and place of determination.
Most contemporary methods meet the above criteria of analytical and clinical precision. Nevertheless, the requirement for harmonized reference/recommended values that could be unquestionably interpreted according to professional recommendations has not been met yet. Actually, despite excellent correlation, results obtained from the same sample and by using different methods exhibit significant differences due to both different analyte determined by different methods (GHb vs HbA1c), and insufficient analytical specificity of methods based on differences in charge (24). Additional issues are analytical and biological interferences that may significantly affect the result. Besides, technological advancement enabled HbA1c determination also outside the laboratory, in physician’s office or even in patient’s hands, often without any awareness of the (un)reliability of thus obtained results.
The consequence of the stated factors is poor comparability of results which, taking into accound the very essence of the clinical application of HbA1c that involves continued, life-long glycemia control, led to serious implications for the quality of diabetologic care and imposed the need for standardization (25).
Standardization programs
American program of standardizing glycohemoglobin determination (26, 27)
In 1996, National Glycohemoglobin Standardization Program (NGSP) was launched in the USA, organized jointly by the American Association of Clinical Chemistry and American Diabetes Association. As a designated comparison method, the method applied in DCCT and UKPDS trials was introduced (high efficiency liquid chromatography on BioRex column). NGSP enables manufacturers to set up result traceability according to DCCT standards via a national reference laboratory. A network of primary and secondary supporting laboratories in the USA and Europe use different methods (chromatography, affinity binding, capillary electrophoresis, and immunochemistry), calibrated according to DCCT traceable values obtained from the National Reference Laboratory. An addition to NGSP is a national external quality control program (CAP Survey; College of American Pathologists), based on interlaboratory exchange of fresh blood samples with HbA1c values declared according to NGSP values. NGSP and CAP Survey have considerably promoted reproducibility of HbA1c determination and interlaboratory harmonization of results and traceability according to DCCT standards. Presently, over 98% of American laboratories meet the prescribed criteria of reproducibility and compliance with DCCT standard, the fact which has resulted in vast progress in the quality of diabetologic care, i.e. application of recommended therapy targets. The basic disadvantage of NGSP is that the designated comparison method, despite exceptional reproducibility, is insufficiently specific to be accepted as a reference method. Thus, despite the attained clinical standardization, the question of analytical standardization has remained unsolved.
IFCC: Working Group on Standardization of HbA1c Determination (28, 29)
International Federation of Clinical Chemistry (IFCC) established in 1995 a Working Group for Standardization of HbA1cDetermination. The analyte was defined as part of this initiative, primary reference material was prepared as a mixture of chromatographically purified HbA1c and HbA0 in lyophilized form, and a reference method was developed and published. It is based on chromatographic separation and identification of b-N-terminal hexapeptides of HbA1c i HbA0 obtained by the action of endoproteinase Glu-C enzyme on intact hemoglobin molecules. It is interesting that two equally efficient systems of identification of peptide fragments were developed, i.e. mass spectrometry and capillary electrophoresis or, actually, two equally valuable reference methods equally specific for HbA1c and insensitive to interferences of abnormal hemoglobins (HbS, HbC) and of post-translationally altered molecules (acetylatedand carbamylatedhemoglobins). The methods are linear for a broad range of clinically relevant values (2.5%–11%) and showed good intra- and interlaboratory reproducibility (CV=1.5%). IFCC program also established a network of reference laboratories (7 European, 2 in Japan and 1 in the USA) which participate in two annual comparison studies with a view to evaluating the stability and mutual traceability of new series of calibrators and control samples, with concurrent laboratory quality control according to the network criteria. Once a year, 8 pool samples of whole blood are analyzed in IFCC network laboratories and handed over to manufacturers for calibration of commercial methods. Surveillance program for manufacturers has also been anticipated.
Despite unquestionable analytical superiority, application of IFCC reference system was rendered impossible by the fact that HbA1c results are significantly lower than NGSP-DCCT traceable values (Table 2).
Table 2 Correlation between NGSP/DCCT and IFCC values of HbA1c
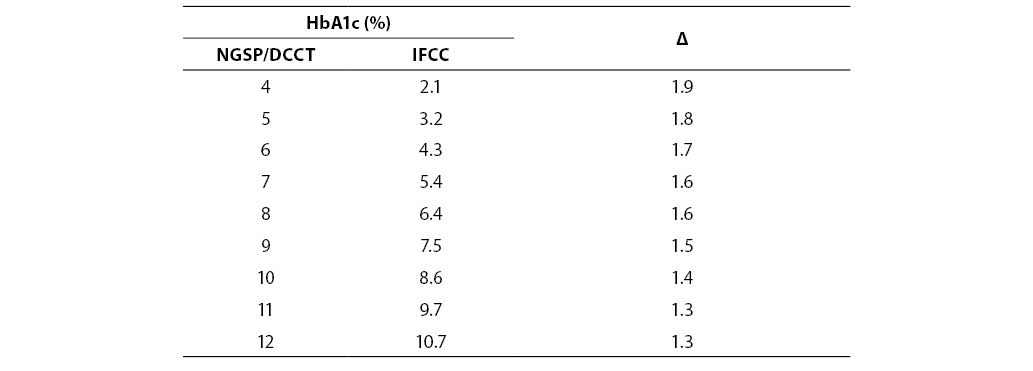
Since international recommendations on good metabolic regulation and reference values for nondiabetic population are both based on DCCT and UKPDS studies, relevant diabetologic organizations (IDF, International Diabetes Federation; ADA; EASD, European Diabetes Study Group; ISPAD, International Society for Pediatric and Adolescent Diabetology) have agreed in a commom conclusion that an abrupt change due to transition to IFCC standardization system would produce unnecessary confusion and might have incalculable clinical, psychological and even financial implications, the more so since IFCC system has not been validated in any clinical study. Therefore, it would be necessary to achieve a broad consensus of clinical and laboratory medicine for application of the IFCC reference system, and this should certainly be achieved on the basis of results of clinical studies. This conclusion indicates the need for extraordinary caution in literal application of regulations by the European Union which, starting from year 2003, committed all manufacturers distributing their reagents in its area to traceability according to the IFCC reference method (22, 23, 30).
Global harmonization project (31, 32)
The task to break a kind of a deadlock between the two systems was conferred to the Working Group of the HbA1c Assay which held its first meeting in London at the beginning of 2004 under the chairmanship of the International Diabetologic Federation (IDF). Representatives of ADA, EASD, IDF, IFCC and NGSP discussed the current status of HbA1c determination, the possibilities created by the development of IFCC reference method, and recommended the manner and scope of its implementation. The following conclusions were reached:
1. IFCC reference method was adopted as a global standard for calibration of all commercial methods.
2. IFCC reference method was adopted as the basis for international certification process in existing networks of reference laboratories.
3. Until further notice, HbA1c results were to be expressed in DCCT equivalents.
Note: Statistical evaluation of comparative results from NGSP and IFCC standardization systems yielded the so-called master formula ŠNGSP = (0.915 × IFCC) + 2.15Ć which allows the transfer of IFCC results into clinically sensible values comparable to DCCT based recommendations, with the possibility for NGSP to apply a higher order system as a basis (29). The master formula was not designed as a basis for application in individual laboratories but it may allow manufacturers to transfer results of the methods, standardized according to the IFCC system (in accordance with EU regulations), into clinically acceptable values.
4. A large-scale project of redefining HbA1c was launched with the view to setting up a newly derived, tentatively termed mean blood glucose.
Members of the Working Group expressed in this conclusion the long observed necessity to meet the need to increase test sensitivity in the minds of diabetic patients. In fact, it has been demonstrated that patients are hard to convince that a relatively slight change in result (e.g., from 7% to 9%) may have vast, in this case disastrous, effect on their health (33). This problem is additionally aggravated by possible transfer to the IFCC system where even 5% HbA1c would imply poor glycemia regulation; in a preliminary trial by Swedish authors, this has to most patients been shown to be an unreasonably low limit that eventually led to poorer metabolic control (34). On the other hand, a recent report has pointed to the possibility of erroneous classification of a significant number of patients (up to 30%) by applying the master formula to convert IFCC results into DCCT equivalents, which additionally supports the idea of preparing a new parameter of mean glycemia (35). Foundation for this initiative was mathematical definition of correlation between HbA1c and mean glycemia which was arrived at by retrospective analysis of 7-point glycemic profile performed within the DCCT study (36):
mean glycemia (MG) = 1.84 x IFCC HbA1c
Provided that the observed correlation is confirmed in prospective studies, there opens up a possibility to redefine mean glycemia measures by converting HbA1c into a value analogous to glucose concentration, with the final aim of setting up a direct and recognizable parameter that would be equally acceptable to both patients and healthcare professionals. This proposal was argumented by stating advantages like clear test revision which excludes the possibility of wrong interpretation, better acceptability and comprehensibility for patients through expanded scale of clinically significant values and potential future test utilization in diagnosis of diabetes.
5. Implementation of the program of educating medical professionals and patients on the new parameter.
The problem in this concept is that past information on the association between HbA1c and mean glycemia indicate high value dispersion (37). Therefore, the studies planned must, above all, elucidate the true character of correlation between these two parameters and the risk for developing diabetic complications in all clinically relevant groups (healthy individuals, pregnant women, diabetic patients of various ethnic background). Of no lesser importance is also the study of potential biological and pharmacological interferences. A multicentric study, with the protocol adopted by the Working Group in June 2005 and an anticipated completion in 2007, should provide answers to all overt issues and the foundation for definitive global harmonization of HbA1c and/or HbA1c-derived parameter of mean glycemia (38, 39).
Where are we?
Laboratory determination of HbA1c has been present in Croatia for 25 years (40). During this period, various analytical methods have been introduced and rigorously evaluated in keeping with global advancement in the field (41–43). However, adequate professional support has been absent at the national level in terms of monitoring and promoting quality and provision of conditions for application of international standards.
The results of a study conducted at the beginning of 2005 demonstrated poor test availability in Croatia (it is performed in only 27 laboratories), and six-fold lower number of determinations compared to recommended requirements for existing diabetic population, which is a decline compared to indicators from year 1999 (44, 14). The methodology is presently rather uniform, with immunoturbidimetric methods applied in as many as 92% of laboratories, but there is a large distribution of reference values that ranged from <5.7% to <7%. Also, it is rather concerning that results in four laboratories (15%) are issued in the form of IFCC equivalents. Study participants almost unanimously expressed their interest in participating in the external quality control program which was still not organized at the time of the survey.
By incorporating HbA1c among general medical biochemistry tests, formal prerequisite was realized for broad test application in primary health care, which is also anticipated by the “Croatian Model of Medical Protection of Diabetic Patients” (45). However, all open questions that encumber analysis and clinical application of HbA1c remained without answers. At the beginning of 2004, a program of standardization of HbA1c determination was launched upon the initiative of the Reference Center for Diabetes of the Republic of Croatia in an attempt to regulate this sensitive problem area at the national level by attainment of the following goals (44):
1. Establish the basis for professional surveillance by setting up a specific module for HbA1c within the framework of the external quality control program by the Croatian Society of Medical Biochemists and by the obligation for all laboratories that determine HbA1c to participate.
2. Ensure continuous information flow through various types of professional education (courses, workshops, publications) as it is important for laboratory experts engaged in HbA1c determination.
3. The data acquired by investigating existing conditions of HbA1c determination in Croatia regarding analytical methodology and clinical utilization of the test should be used not only for educational purposes but also as an argument in negoations during contracting this test at the primary health care level immediately after setting up the mechanisms of continuous education and professional surveillance described in previous sections.
4. Ensure rigorous application of internationally accepted standards for HbA1c determination through a system of documents by the Croatian Chamber of Medical Biochemists related to laboratory practice standards in Croatia.
The objectives of the national program for HbA1c determination are already in the process of implementation. Recommendations have been defined for determination procedures in medical biochemistry laboratories (46), continuous education program was implemented (47), and a (too) long missing external quality control program has been launched (Module 8 of the external quality control program by the Croatian Society of Medical Biochemists).
Conclusion
Changes in laboratory methods and/or reference intervals represent a common practice and mostly do not cause disturbances in healthcare quality. However, HbA1c is a specific exception as it is not used only by medical professionals but also by both patients and their families as a powerful means of collaboration in achieving therapeutic goals (48). Although analytical harmonization, i.e. standardization of results by applying precise reference methodology and defined reference material, is necessary, its implementation must be consistent with clinical standards and guidelines based on precise yet insufficiently specific analytical methodology. In case of HbA1c, laboratory medicine should have acknowledged the fact that laboratories, working groups for standardization and metrologic institutions do not exist as an end in themselves but serve to accomplish the goals of a specific branch of clinical medicine, in this case diabetology.
The global project of harmonization of HbA1c determination is presently indeed a major task for clinical and laboratory professions involved in diabetologic care. All relevant international institutions are involved in execution of this task, which, on one hand, indicates the importance of solving this issue and, on the other, provides a guarantee that the goal will be realized, i.e. that a unique parameter and measure will be established that will, with highest reliability, provide objective insight into glycemia control. Medical and biochemical profession in Croatia has in this, as well as in other fields, full responsibility for continuous follow-up, adoption and application of international standards.
References
1. King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2005: prevalence, numerical estimates and projections. Diab Care 1998;21:1414-31.
2. Metelko Ž, Pavlić-Renar I, Poljičanin T, Szirovicza L, Turek S. Prevalencija šećerne bolesti u Hrvatskoj. Knjiga sažetaka II. hrvatskog epidemiološkog kongresa, Rovinj, 26.-29.10.2005. (in press).
3. Amos AF, McCarthy DJ, Zimmet P. The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diab Med 1997;14:S7-45.
4. Sandberg S. What is the global campaign? An overview with emphasis on self-measurement of blood glucose and patient needs. Clin Chim Acta 2005;355 (Supl): S40.
5. American Diabetes Association. Standards of medical care for diabetes. Diab Care 2004;27(Suppl 1)S15-35.
6. DCCT Study Group. The effect of intensive therapy of diabetes on the development and progression of long-term complications of insulin-dependent diabetes mellitus: the Diabetes Control and Complications Trial. N Engl J Med. 1993;329:977-86.
7. UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk for complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352: 837-53.
8. Goldstein DE, Little RR, Wiedmeyer H-E, England JD, Rohlfing CL, Wilke AL. Is glycohemoglobin testing useful in diabetes mellitus? Lessons from the Diabetes Control and Complications Trial. Clin Chem 1994;40:1637-40.
9. Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA et al. Association of glycaemia with microvascular and macrovascular complications of type 2 diabetes (UKPDS 35): prospective observational study. Br Med J 2000; 321:405-12.
10. Marshall SM, Home PD, Manley SE, Barth JH, John WG. Standardisation of glycated hemoglobin. Ann Clin Biochem 2002;39:78-9.
11. John WG. Haemoglobin A1c: Analysis and standardisation. Clin Chem Lab Med 2003;41:1199-212.
12. Rohlfing C, Wiedmeyer HM, Little R, Grotz LV, Tennill A, England J, Madsen R, Goldstein D. Biological variation of glycohemoglobin. Clin Chem 2002;48:1116-8.
13. Jeffcoate SL. Diabetes control and complications: the role of glycated haemoglobin, 25 years on. Diabetic Medicine 2003;21:657-65.
14. Vučić M, Božičević S, Mesić R, Ročić B, Metelko Ž. Implications of the glycohemoglobin/HbA1c testing for health care of patients with diabetes mellitus. Diabetol Croat 1999;28:173-8.
15. Pravilnik o vrstama pretraga koje obavljaju medicinsko-biokemijski labratoriji. Narodne novine 2003;197:3150
16. Allen DW, Schroeder WA, Balog J. Observations on the chromatographic heterogneity of normal adult and fetal human hemoglobin. J Am Chem Soc 1958; 80:1628-34.
17. Rahbar S. An abnormal hemoglobin in red cells of diabetics Clin Chem Acta 1968; 22:296-8.
18. Trivelli LA, Ranney HM, Lai H-T. Hemoglobin components in patients with diabetes mellitus. N Engl J Med 1971;248:353-7.
19. Leslie RGD, Pyke DA, John PN, White JM. Fast glycosylation of glucose. Lancet 1979;i:773-4.
20. Bunn HF, Haney DN, Kamin S, Gabbay KH, Gallop PM. The biosynthesis of human hemoglobin A1c. J Clin Invest 1976;57:1652-9.
21. Baynes JW, Monnier VM, ur. The Maillard reaction in ageing, diabetes and nutrition. New York: Liss, 1989.
22. Goldstein DE, Little RR, Lorenz RA, Malone JI, Nathan D, Peterson CM, Sacks DB. Tests of glycemia in diabetes. Diabetes Care 2004;27:1761-73.
23. Sacks DB, Bruns DE, Goldstein DE, Maclaren NK, McDonald JM, Parrott M. Giudelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem 2002;48:436-72.
24. Little R. Recent progress in glycohemoglobin (HbA1c) testing. Diab Care 2000;23: 265-6.
25. Boulton AJ, Saudek CD. The need for standardisation of glycated hemoglobin measurements. Diabetologia 2002; 45:R19-21.
26. Little RR, Rohlfing CL, Wiedmeyer HM, Myers GL, Sachs DB, Goldstein DE. The National Glycohemoglobin Standardization Program: a five-year progress report. Clin Chem 2001;47:1985-92.
27. Little RR. Glycated hemoglobin standardization – National Glycohemoglobin Standardization Program (NGSP) perspective. Clin Chem Lab Med 2003;41: 1191-8.
28. Jeppson J-O, Kobold U, Barr J, Finke A, Hoelzel W, Hoshino T, et al. Approved IFCC reference method for the measurement of HbA1c in human blood. Clin Chem Lab Med 2002;40:78-89.
29. Hoelzel W, Weykamp C, Jeppson J-O, Miedema K, Barr J, Goodall I, et al. On behalf of the IFCC Working Group on HbA1c standardization. The IFCC reference system for the measurement of HbA1c in human blood and the national standardization schemes in the USA, Japan and Sweden. Clin Chem 2004;50: 166-74.
30. Manley S, John GW, Marshall S. Introduction of IFCC reference method for calibration of HbA1c: Implications for clinical care. Diab Med. 2004;21:673-6.
31. Report of the ADA/EASD/IDF Working Group of the HbA1c assay. Diabetologia 2004;47:R53-4.
32. Minutes of the follow-up meeting of the ADA/EASD/IDF Working Group of the HbA1c assay. Diabetologia 2005;48:R14-6.
33. Larsen ML, Horder M, Magensen EF. Effect of long-term monitoring of glycosylated hemoglobin levels in insulin-dependent diabetes mellitus. N Engl J Med 1990;323:1021-5.
34. Hanas R. Psychological impact of changing the scale of reported HbA1c results affects metabolic control. Diab Care 2002;25:2110-1.
35. Singh Datt G, Agarwal MM, Bishawi B. HbA1c: A comparison of NGSP with IFCC transformed values. Clin Chim Acta 2005;358:81-6.
36. Rohlfing CL, Wiedmeyer H-M, Little RR, England JD, Tennill A, Goldstein DE. Defining the relationship between plasma glucose and HbA1c: analysis of glucose profiles and HbA1c in the Diabetes Control and Complications Trial. Diab Care 2002;25:275-8.
37. Miedema K. Towards worldwide standardization of HbA1c determination. Diabetologia 2004;47:1143-8.
38. Sacks DB. Global harmonization of hemoglobin A1c. Clin Chem 2005;51:681-3.
39. Goodall I. HbA1c Standardisation. Destination – global IFCC standardisation. How, why, where and when. Clin Biochem Rev 2005;26:5-20.
40. Topić, E. Glikozilirani hemoglobini i šećerna bolest. Disertacija, Sveučilište u Zagrebu, 1981.
41. Topić E, Zadro R, Granić M, Škrabalo Z. Filter paper blood sampling for glycated haemoglobin determination and its use in the control of diabetes mellitus. J Clin Chem Clin Biochem 1987;25:261-4.
42. Breyer D, Mesić R, Ročić B. Application of the FPLC system for the determination of HbA1c. Precision, sampling and stability study. Diabetol Croat 1990;19:225-8.
43. Vučić M, Petrović S, Mesić R, Ročić B. An automated immunoturbidimetric assay for HbA1c determination. Diabetol Croat 1998;27:85-90.
44. Vučić Lovrenčić M, Božičević S, Juretić D, Topić E. Program standardizacije određivanja hemoglobina A1c u Hrvatskoj. Liječnički vjesnik 2005;127(Suppl 1):83.
45. Metelko Ž, Babić Z, Car N, Pavlić-Renar I; Ročić B; Škrabalo Z, Granić M. The Croatian model of diabetes care and St. Vincent declaration. Diabetes, Nutrition & Metabolism - Clinical & Experimental 2000;13:178-80.
46. Povjerenstvo za harmonizaciju laboratorijskih nalaza HKMB. Harmonizacija laboratorijskih nalaza u području opće medicinske biokemije. HKMB, 2004.
47. Vučić M, Metelko Ž. Standardizacija određivanja hemoglobina A1c: analitičke i kliničke implikacije. Priručnik edukacijskog programa/radionice za medicinske bikemičare. Sveučilišna klinika Vuk Vrhovac, 2005.
48. Holt RIG, Gallen I. Time to move beyond glycosylated haemoglobin. Diabet Med 2004;21:655-6.