For more information on Publication Ethics and Research Integrity issues please see articles published as a part of the Biochemia Medica's Research Integrity Corner.
The Editorial board of Biochemia Medica strongly promotes research integrity and aims to prevent any type of scientific misconduct and questionable research practice, such as fabrication, falsification, plagiarism, redundant publication, and authorship problems. All submitted manuscripts are revised by Research Integrity Editor and checked using Crossref Similarity Check (powered by iThenticate) screening system for potential plagiarism. Very basic definition of plagiarism given by the Office of Research Integrity (ORI) is “theft or misappropriation of intellectual property that includes unauthorized use of ideas or unique methods obtained by a privileged communication, such as a grant or manuscript review“. However, apart from blatant plagiarism there are many forms of misappropriation of other's ideas (patchwork plagiarism, technical plagiarism etc.) or recycling one's own text or ideas (salami publication, duplicate publication etc.). Therefore, all manuscripts are additionally checked for redundant publication by searching authors previously published work. If duplicate or salami publication is suspected, Editors will ask the authors for explanation. In resolving any potential scientific misconduct (detected prior or after publication), Biochemia Medica follows flowcharts provided by the Committee on Publication Ethics (COPE) and additionally consults COPE for any unclear cases.
More detailed editorial policies regarding other research integrity issues are published in Biochemia Medica’s special section called Research integrity corner. All authors are invited to contribute by preparing manuscripts for this section regarding any issue important for promotion of research ethics and integrity. Furthermore, we encourage all our readers, reviewers, and authors to report any misconduct or questionable research practice should they encounter one when reading of reviewing manuscripts in Biochemia Medica. Editors of Biochemia Medica are dedicated to investigating all raised concerns about submitted and published articles according to the protocol for dealing with allegations of misconduct given by the COPE (available at: https://publicationethics.org/misconduct). During the investigation, in order to be more effective, Editors of Biochemia Medica can contact other journals’ Editors involved in the case. In order to preserve highest ethical standards of editorial practice Editors of Biochemia Medica follow COPE recommendations for sharing the information among Editors (available at: https://publicationethics.org/resources/guidelines-new/sharing-information-among-editors-chief-regarding-possible-misconduct). Furthermore, Editors of Biochemia Medica are aware of the possibility to receive concerns about any research aspect by so called whistle-blowers. In the respect of resolving any raised issue we follow flowcharts given by the COPE for this specific situation: “Responding to whistle-blowers when concerns are raised directly” and “Responding to whistle-blowers when concerns are raised via social media”.
Article retractions or expressions of concern
If editorial investigation according to all relevant recommendations proves misconduct in the published article and if the article fulfils one of the listed reasons for retraction given by the COPE’s “Retraction guidelines” than the Editors of Biochemia Medica will issue a Notice of retraction. Notice of retraction will be published according to the recommendation given in the COPE’s document and in respect to the checklist for retraction given by the European Association of Science Editors (EASE). If there is no strong evidence for retraction but some concerns can be raised than an Expression of concern may be published.
IMPORTANT: Article corrections published as Corrigendum or Erratum are not in any way questionable research practices but corrections of unintentional mistakes made either by the authors of the article or by the Journal's editors during the manuscript editing process. If author correction are needed after publication, a request indicating the correction(s) needed should be submitted by the corresponding author to the Editorial office of the Journal. Editors will review the corrections requested according to the recommendations issued by ICMJE. If acceptable, the corrections will be published in the form of Erratum or Corrigendum as soon as possible, referring to the original publication.
Authorship
Biochemia Medica adheres to guidelines for authorship set forth by the International Committee of Medical Journal Editors (ICMJE).
Each author should meet all four criteria as follows:
According to ICMJE: “In addition to being accountable for the parts of the work the author has done, an author should be able to identify which co-authors are responsible for specific other parts of the work. In addition, authors should have confidence in the integrity of the contributions of their co-authors. All those designated as authors, should meet all four criteria for authorship, and all who meet the four criteria should be identified as authors.“ Those who made substantial contribution and meet all four authorship criteria but are not listed as authors are called ghost authors, whereas those who are listed as authors, despite their negligible contribution, are called honorary authors (guest authors or gift authors). Biochemia Medica does not allow honorary authorship. Instead, all who have made substantial contributions to the work but do not meet the criteria for authorship should be listed in the Acknowledgments section (technical help, writing assistance, language translation service, general support, financial and material support). All contributors named in the Acknowledgments section of the manuscript must give their permission to be named. Statement for such permission is included in the manuscript submission process.
Biochemia Medica has adopted the CRediT taxonomy (Contributor Roles Taxonomy) for recognition of contributor roles with the intention of better transparency and avoiding possible authorship disputes. CRediT offers authors the opportunity to uniformly share an accurate and detailed description of their diverse contributions to the published work. It is the responsibility of the corresponding author to ensure that the descriptions are accurate and agreed by all contributors. CRediT taxonomy defines 14 different contributor roles that cover all aspects of the study from the first concept to article publication. Each author’s contribution has to be defined by CRediT term that best describes his role in the study. Authors may have multiple roles and multiple authors may have the same role. CRediT statements for every individual author are provided during the submission process and will appear in a separate section of the published article. Please keep in mind that the authorship is still based on the above stated four ICMJE criteria.
In dealing with authorship disputes Biochemia Medica follows COPE guidelines and flowcharts.
Biochemia Medica has adopted the ORCID system by which each author is identified with a unique identification number. ORCID ensures transparency in authorship and personal identification. It is easily obtained and available.
If any changes in the authors list is made after initial submission or during revision of the manuscript, the corresponding author should contact the Editorial office of the Journal and explain the reasons for the change. All changes in the author list, including additions, deletions, order change or contributions differently attributed, must be approved by all authors. In resolving problems that may arise from authors’ list changes, Biochemia Medica is guided by COPE flowcharts.
For more information on Biochemia Medica’s policy on authorship, readers and authors are encouraged to read the article Šupak-Smolčić V, Šimundić AM. Biochemia Medica’s editorial policy on authorship. Biochem Med (Zagreb) 2015;25(3):320-3.
Ethical approval and informed consent
When reporting trials on human subjects, authors should indicate whether the procedures were in accordance with the ethical standards set by the responsible human experimentation committee (institutional and national) and latest version of the Declaration of Helsinki given by World Medical Association. Ethical approval (institutional or national) should be obtained for every study that includes collection of additional patient sample of any biological material (more than those required for the medical evaluation). Information on ethical approval should be stated in the manuscript.
All subjects should sign an informed consent form and this information should be provided in the manuscript. Signed informed consent forms should be archived by the authors. The authors have to provide a statement that they have received and archived all patient informed consent forms, as required during the manuscript submission process. It should be noted that informed consent to participate in the research does not imply consent to publish personal individual data (names, pictures, hospital identification). Therefore, for publication that includes any individual data, patients must sign an informed consent. This is especially applied when it is not possible to obtain anonymity of the data without distorting scientific evidence.
Regardless of the preserved anonymity, patients presented in case report articles should always sign informed consent. Case reports without patients’ consent are not eligible for publication in Biochemia Medica. Specific types of case reports, Preanalytical mysteries, are not obliged to obtain informed consent if there are no patient's personal data revealed. If there is need to publish patient's rare diagnosis or specific demographic or personal data by which patient's identity can be implied, then the authors must obtain patient's signed informed consent.
In the spirit of promoting best practice guidelines given by COPE, Biochemia Medica will not consider for publication manuscripts in which the best ethical practice is not ensured, i.e. informed consent is missing and/or ethical approval is omitted.
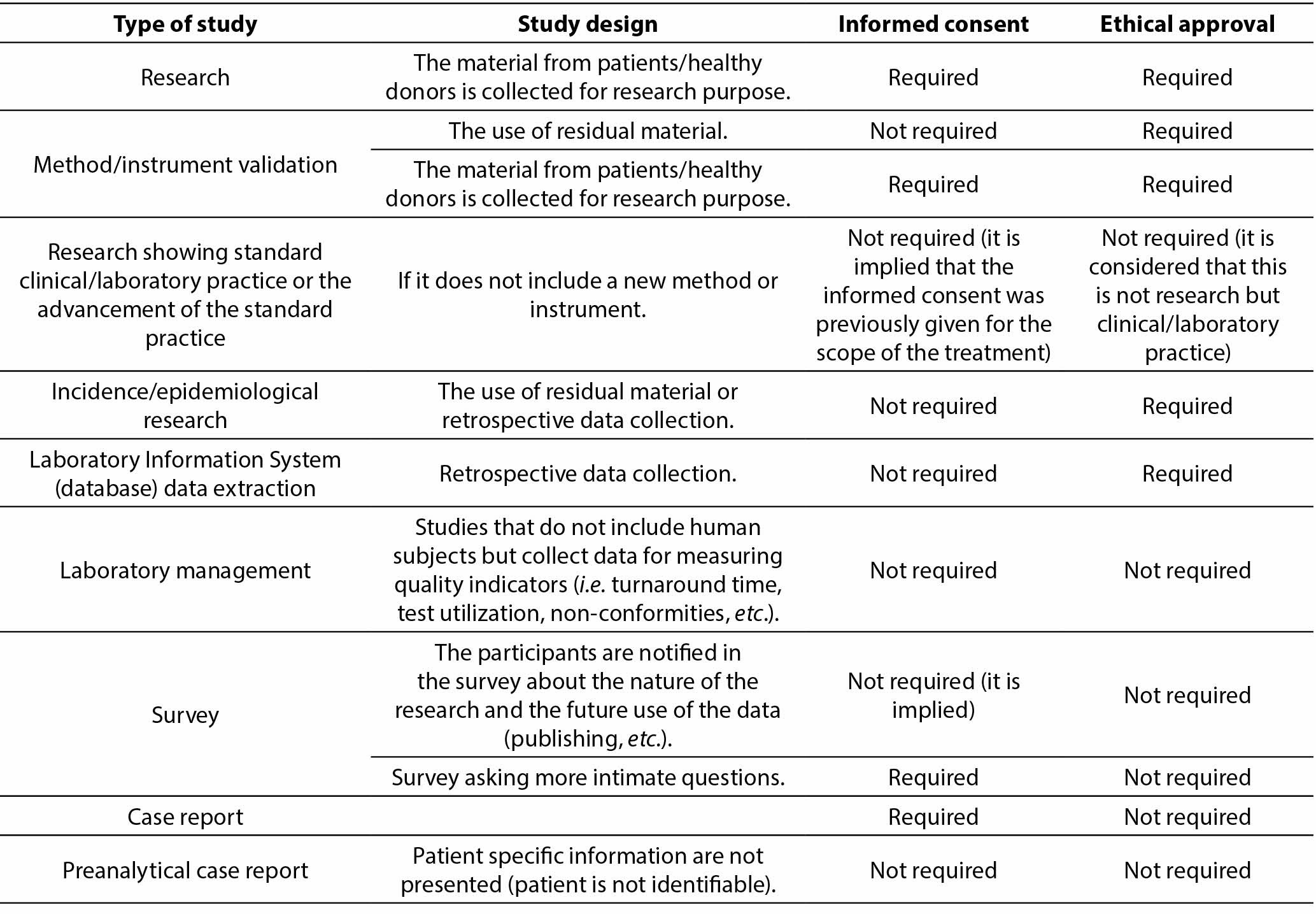
To simplify the decision-making process on whether a type of study requires informed consent and/or ethical approval, authors are encouraged to consult the table below reprinted from Borovecki A, Mlinaric A, Horvat M, Supak Smolcic V. Informed consent and ethical approval in laboratory medicine. Biochem Med (Zagreb) 2018;28(3):030201.
Clinical trial registration and data sharing
Biochemia Medica will take into consideration manuscripts dealing only with registered clinical trials in a public trial registry. Clinical trials have to be registered before first patient was included in the study. Since clinical trials are interventional research studies involving humans, it is necessary to obtain highest degree of ethical standards and transparency and responsible research conduct. Furthermore, registration of clinical trial prevents any misuse or abuse of study data. Biochemia Medica endorses recommendation on clinical trials given by the ICMJE. More detailed information about the background on clinical trial registration are available at: http://www.icmje.org/recommendations/browse/publishing-and-editorial-issues/clinical-trial-registration.html.
There are numerous clinical trials registries and Biochemia Medica will give advantage to those which are registered in registries recommended by ICMJE because of more comprehensive and independent criteria for registration of trials. List of registries is available at: http://www.icmje.org/about-icmje/faqs/clinical-trials-registration/.
In respect to open science and complete transparency of research conduct, Biochemia Medica strongly encourages all authors to provide data collected during their study (i.e. data on which their results are based and conclusions drawn). There are numerous free repositories and Biochemia Medica does not endorse any but prefers those that can be connected to specific content using DOI number or to specific author ORCID related. Few examples of data repositories are:
Dryad Digital Repository (https://datadryad.org/stash)
Code ocean (https://codeocean.com/)
Zenodo (https://zenodo.org/)
Figshare (https://figshare.com/)
Harvard Datavers (https://dataverse.harvard.edu/)
DABAR (https://dabar.srce.hr/dabar)
For more information on specific requirements on data sharing please consult https://fairsharing.org/ or https://www.re3data.org/.
Authors should provide a Data availability statement during manuscript submission and this statement will be published at the end of the article. Authors should not incorporate this statement in the manuscript because it could impair anonymity of double blind peer-review. If for some reason authors are not able to share their study data, they should provide an explanation in the Data availability statement. Few examples of Data availability statements can be found at: https://www.springernature.com/gp/authors/research-data-policy/data-availability-statements/12330880
Copyright and publication license
After a manuscript is accepted for publication, the authors must guarantee that all copyrights to the manuscript are transferred to Biochemia Medica. The publisher (Croatian Society of Medical Biochemistry and Laboratory Medicine) has the right to reproduce and distribute the article in printed and electronic form without asking permission from authors. All manuscripts published online are subject to Creative Commons Attribution License CC-BY which permits users to read, download, copy, distribute, print, search, or link to the full texts of these articles in any medium or format. Also, users can remix, transform and build upon the material, provided the original work is properly cited and any changes properly indicated. Complete legal background of license is available at: https://creativecommons.org/licenses/by/4.0/legalcode.
Biochemia Medica requires authors to obtain and acknowledge copyright permission to use, reproduce or adapt any copyrighted material (i.e. figures, research tools) from another source (copyright holder).
Archiving policy
Biochemia Medica archives all submitted manuscripts whether rejected or accepted and keeps them strictly confidential. The authors have the right to archive the accepted version of their work (postprint) or the final published article (version of record) in an institutional website, personal website, public repository or any other form of archive, provided the original work is properly cited and any changes properly indicated.
Biochemia Medica will consider for publication any manuscript that is in the Journal’s scope and prepared according to the Instructions for authors regardless of its existence in preprint database. Biochemia Medica does not consider trial preregistration, conference abstract or presentation as prepublication of submitted manuscript. However, authors should declare all accessible work that is connected to their manuscript.
Conflict of interest
Biochemia Medica encourages all authors and reviewers to report any potential conflicts of interest to ensure complete transparency regarding the preparation and reviewing the manuscript (research funding, grants, sponsorship, competing interests etc.). According to the International Committee of Medical Journal Editors (ICMJE): “Conflict of interest exists when an author (or the author’s institution) has financial (employment, consultancies, stock ownership, honoraria and paid expert testimony) or personal relationship, academic competition or intellectual passion that inappropriately influences his actions.”
Authors are required to confirm that all applicable conflicts of interest are declared during the manuscript submission process. A conflict of interest statement should be stated on the Title page of each submitted manuscript and will be published at the end of the manuscript. Reviewers are asked to report conflict of interest when responding to the invitation by sending short statement to the editorial office. If conflict of interest appears during review process, reviewers should report them accordingly.
Good editorial practice of Biochemia Medica journal implies unbiased editorial decisions. If an Editor feels that his judgement will not be objective due to potential conflict of interest, then he will excuse himself from further editorial decisions. Additionally, editors will never use any information they acquired during their editorial work. Each Editor of Biochemia Medica as well as guest editors should sign conflict of interest statement.
In resolving specific issues regarding undisclosed conflict of interest in submitted or published manuscript, Biochemia Medica consults relevant COPE flowcharts.
Declaration of generative AI in scientific writing
Biochemia Medica fully adopts COPE statement on use of artificial intelligence (AI) and AI-assisted technologies in scientific publishing. For more information, please visit: https://publicationethics.org/guidance/cope-position/authorship-and-ai-tools.
Authors are allowed to use generative AI and AI-assisted technologies during the writing process only to improve readability and language.
Artificial intelligence and AI-assisted technologies should not be listed as an author or co-author, or be cited as an author. Authorship implies responsibilities and tasks that can only be attributed to and performed by humans.
Authors should disclose in their manuscript the use of AI and AI-assisted technologies in the writing process in the Materials and methods section by citing the name, version and source of the AI-assisted technologies. A statement will appear in the published work. Please note that authors are ultimately responsible and accountable for the contents of the work.
This declaration does not apply to the use of basic tools for checking grammar, spelling, references etc. If there is nothing to disclose, there is no need to add a statement.