References
1. Smirnoff N. L-ascorbicacid biosynthesis. Vitam Horm 2001;61:241-66.
2. Kaegi E. Unconventional therapies for cancer: Vitamins A, C and E. Can Med Assoc J 1998;158(11):1483-8.
3. Pauling L. Ascorbic acid and the common cold. Am J Clin Nutr 1971;24(11):1294-9.
4. Cameron E, Pauling L. Supplemental ascorbate in the supportive treatment of cancer: reevaluation of prolongation of survival times in terminal human cancer. Proc Natl Acad Sci U S A 1978;75(9):4538-42.
5. Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR, et al. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc Natl Acad Sci USA 1996; 93(8):3704-9.
6. Food and Nutrition Board, Institute of Medicine, US National Academy of Science. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. National Academy Press, Washington; 2000, pp 95-185.
7. Carr AC, Frei B. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am J Clin Nutr 1999;69(6):1086-107.
8. Levine M, Rumsey SC, Daruwala RC, Park JB, Wang Y. Criteria and recommendations for vitamin C intake. JAMA 1999;281(15):1415-23.
9. Kojo S. Vitamin C: basic metabolism and its function as an index of oxidative stress. Curr Med Chem 2004;11(8):1041-64.
10. Buettner GR. The pecking order of free radicals, antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbate. Arch Biochem Biophys 1993;300(2):535-43.
11. Padayatty SJ, Levine M. New insights into the physiology and pharmacology of vitamin C. Canad Med Assoc J 2001;164(3):353-5.
12. Patak P, Willenberg HS, Bornstein SR. Vitamin C is an important cofactor for both adrenal cortex and adrenal medulla. Endocr Res 2004;30(4):871-5.
13. Rumsey SC, Levine M. Absorption, transport, and disposition of ascorbic acid in humans. J Nutr Biochem 1998;9:116-30.
14. Rumsey SC, Kwon O, Xu GW, Burant CF, Simpson I, Levine M. Glucose transporter isoforms GLUT1 and GLUT3 transport dehydroascorbic acid. J Biol Chem 1997;272:18982-9.
15. Tsukaguchi H, Tokui T, Mackenzie B, Berger UV, Chen XZ, Wang Y, et al. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature 1999;399:70-5.
16. Bornstein SR, Yoshida-Hiroi M, Sotiriou S, Levine M, Hartwig HG, Nussbaum RL, et al. Impaired adrenal catecholamine system function in mice with deficiency of the ascorbic acid transporter (SVCT2). FASEB J 2003;17(13):1928-30.
17. Bors W, Buettner GR. The vitamin C radical and its reactions. In: Vitamin C in Health and Disease. Packer L, Fuchs J, eds. New York: Marcel Dekker; 1997; 75-94.
18. Sowell J, Frei B, Stevens JF. Vitamin C conjugates of genotoxic lipid peroxidation products: structural characterization and detection in human plasma. Proc Natl Acad Sci U S A 2004;101(52):17964-9.
19. Halliwell B, Gutteridge JMC. Ascorbic acid. In: Free radicals in biology and medicine. 3rd ed. Oxford Press, Oxford; 1999;200-8.
20. Ahmad IM, Aykin-Burns N, Sim JE, Walsh SA, Higashikubo R, Buettner GR, et al. Mitochondrial O2*- and H2O2 mediate glucose deprivation-induced stress in human cancer cells. J Biol Chem 2005;280(6):4254-63.
21. Halliwell B. The antioxidant paradox. Lancet 2000;355(9210):1179-80.
22. Bijur GN, Briggs B, Hitchcock CL, Williams MV. Ascorbic acid-dehydroascorbate induces cell cycle arrest at G2/M DNA damage checkpoint during oxidative stress. Environ Mol Mutagen 1999;33(2):144-52.
23. Lenton KJ, Therriault H, Fulop T, Payette H, Wagner JR. Glutathione and ascorbate are negatively correlated with oxidative DNA damage in human lymphocytes. Carcinogenesis 1999;20(4):607-13.
24. Podmore ID, Griffiths HR, Herbert KE, Mistry N, Mistry P, Lunec J. Vitamin C exhibits pro-oxidant properties. Nature 1998;392:559-66.
25. Crott JW, Fenech M. Effect of vitamin C supplementation on chromosome damage, apoptosis, necrosis ex vivo. Carcinogenesis 1999;20(6):1035-41.
26. Antunes LM, Takahashi C. Effects of high doses of vitamins C and E against doxorubicin-induced chromosomal damage in Wistar rat bone marrow cells. Mutat Res 1998;419(1-3):137-43.
27. Ames BN. DNAdamage from micronutrient deficiencies is likely to be a major cause of cancer. Mutat Res 2001;475(1-2):7-20.
28. Scarpa M, Stevanato R, Vigilino P, Rigo A. Superoxide ion as active intermediate in the auto oxidation of ascorbate by molecular oxygen. Effect of superoxide dismutase. J Biol Chem 1983;258(11):6695-7.
29. Halliwell B, Gutteridge JMC. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol 1990;186:1-85.
30. Burkitt MJ, Duncan J. Effects of trans-resveratrol on copper-dependent hydroxyl-radical formation and DNA damage: evidence for hydroxyl-radical scavenging and a novel, glutathione-sparing mechanism of action. Arch Biochem Biophys 2000;381(2):253-63.
31. McCord M. The evolution of free radicals and oxidative stress. Am J Med 2000;108(8):652-9.
32. Shankaran M, Yamamoto BK, Gudelsky GA. Ascorbicacid prevents 3,4-methylenedioxymethamphetamine (MDMA)-induced hydroxylradical formation and the behavioral and neurochemical consequences of the depletion of brain 5-HT. Synapse 2001;40(1):55-64.
33. Rehman A, Collis CS, Yang M, Kelly M, Diplock A T, Halliwell B, Rice-Evans E. The effects of iron and vitamin C co-supplementation on oxidative damage to DNA in healthy volunteers. Biochem Biophys Res Commun 1998;246:293-8.
34. Premkumar K, Bowlus CL. Ascorbic acid does not increase the oxidative stress induced by dietary iron in C3H mice. J Nutr 2004;134(2):435-8.
35. Suh J, Zhu BZ, Frei B. Ascorbate does not act as a pro-oxidant towards lipids and proteins in human plasma exposed to redox-active transition metal ions and hydrogen peroxide. Free Radic Biol Med 2003;34(10):1306-14.
36. Church DF, Pryor WA. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ Health Perspect 1985;64:111-26.
37. Gackowski D, Kowalewski J, Siomek A, Olinski R. Oxidative DNA damage and antioxidant vitamin level: comparison among lung cancer patients, healthy smokers and nonsmokers. Int J Cancer 2005;114(1):153-6.
38. Chen HW, Chien ML, Chaung YH, Lii CK, Wang TS. Extracts from cigarette smoke induce DNA damage and cell adhesion molecule expression through different pathways. Chem Biol Interact 2004;150(3):233-41.
39. Takase B, Etsuda H, Matsushima Y, Ayaori M, Kusano H, Hamabe A, et al. Effect of chronic oral supplementation with vitamins on the endothelial function in chronic smokers. Angiology 2004;55(6):653-60.
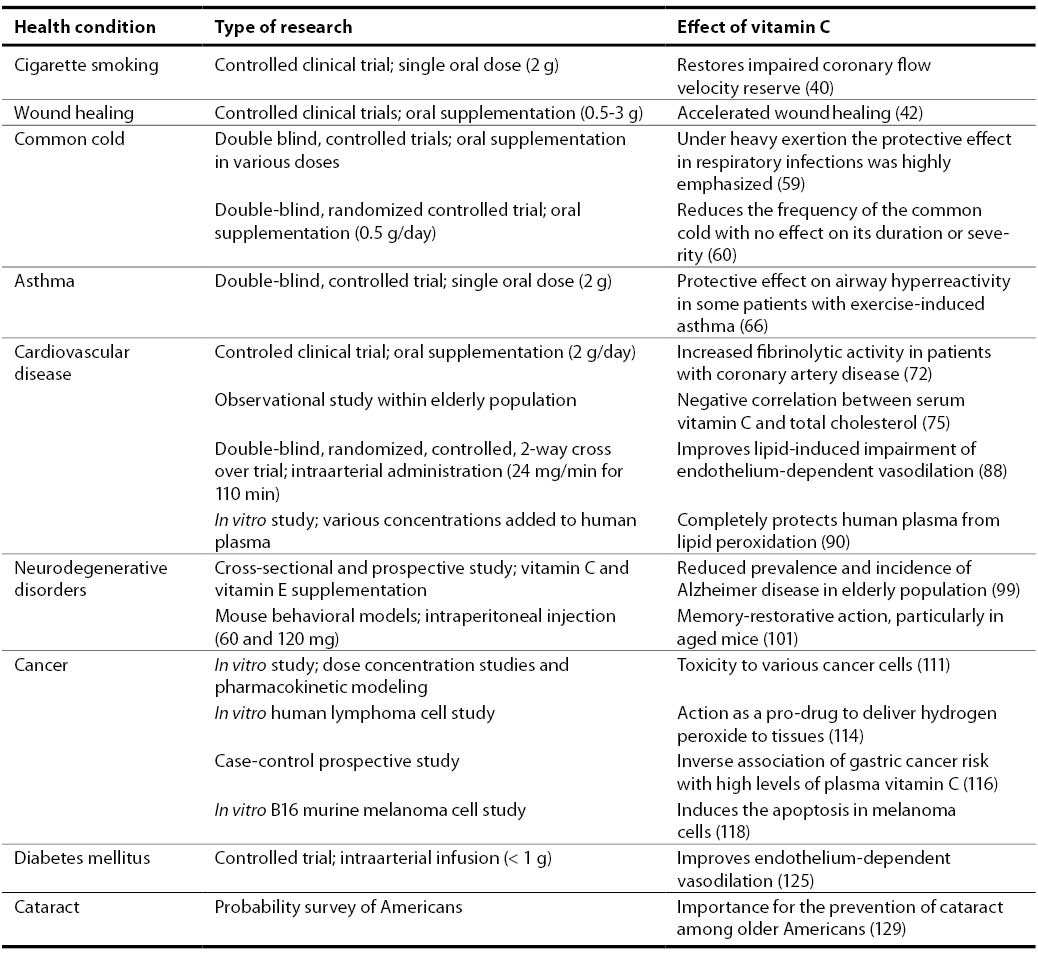
40. Teramoto K, Daimon M, Hasegawa R, Toyoda T, Sekine T, Kawata T, Yoshida K, Komuro I. Acute effect of oral vitamin C on coronary circulation in young healthy smokers. Am Heart J 2004;148(2):300-5.
41. Hemila H. Vitamin C and the common cold. Brit J Nutr 1992;67:3-16.
42. Ringsdorf WM Jr, Cheraskin E. Vitamin C and human wound healing. Oral Surg Oral Med Oral Pathol 1982;53(3):231-6.
43. Wha Kim S, Lee IW, Cho HJ, Cho KH, Han Kim K, Chung JH, et al. Fibroblasts and ascorbate regulate epidermalization in reconstructed human epidermis. J Dermatol Sci 2002;30(3):215-23.
44. Freyria AM, Ronziere MC, Roche S, Rousseau CF and Herbage D. Regulation of growth, protein synthesis, and maturation of fetal bovine epiphyseal chondrocytes grown in high-density culture in the presence of ascorbic acid, retinoic acid, and dihydrocytochalasin. B J Cell Biochem 1999;76:84–98.
45. Sarisozen B, Durak K, Dincer G, Bilgen OF. The effects of vitamins E and C on fracture healing in rats. J Int Med Res 2002;30(3):309-13.
46. Hughes DA. Effects of dietary antioxidants on the immune function of middle-aged adults. Proc Nutr Soc 1999;58:79-84.
47. Del Rio M, Ruedas G, Medina S, Victor VM, De La Fuente M. Improvement by several antioxidants of macrophage function in vitro. Life Sci 1998;63:871-81.
48. Schwager J, Schuize J. Modulation of interleukin production by ascorbic acid. Vet Immunol Immunopathol 1998;64:45-57.
49. Jeng KC, Yang CS, Siu WY, Tsai YS, Liao WJ, Kuo JS. Supplementation with vitamins C and E enhances cytokine production by peripheral blood mononuclear cells in healthy adults. Am J Clin Nutr 1996;64:960-5.
50. Will JC, Byers T. Does diabetes mellitus increase the requirement for vitamin C? Nutr Rev 1996;54(7):193-202.
51. Bonham MJ, Abu-Zidan FM, Simovic MO, Sluis KB, Wilkinson A, Winterbourn CC, et al. Early ascorbic acid depletion is related to the severity of acute pancreatitis. Brit J Surg 1999;86(10):1296-1301.
52. Vural H, Uzun K. Serum and red blood cell antioxidant status in patients with bronchial asthma. Can Respir J 2000;7(6):476-80.
53. Vita JA, Keaney JF Jr, Raby KE, Morrow JD, Freedman JE, Lynch S, et al. Low plasma ascorbic acid independently predicts the presence of an unstable coronary syndrome. J Am Coll Cardiol 1998;31(5):980-6.
54. Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O, Lee JH, et al. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr 2003;22(1):18-35.
55. Audera C, Patulny RV, Sander BH, Douglas RM. Mega-dose vitamin C in treatment of the common cold: a randomised controlled trial. Med J Aust 2001;175(7):359-62.
56. Gorton HC, Jarvis K. The effectiveness of vitamin C in preventing and relieving the symptoms of virus-induced respiratory infections. J Manipulative Physiol Ther 1999;22(8):530-33.
57. Douglas RM, Chalker EB, Treacy B. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev 2004;(2):CD000980.
58. Hemila H, Douglas RM. Vitamin C and acute respiratory infections. Int J Tuberc Lung Dis 1999;3(9):756-61.
59. Hemila H. Vitamin C supplementation and respiratory infections: a systematic review. Mil Med 2004;169(11):920-5.
60. Sasazuki S, Sasaki S, Tsubono Y, Okubo S, Hayashi M, Tsugane S. Effect of vitamin C on common cold: randomized controlled trial. Eur J Clin Nutr 2006;60(1):9-17.
61. Smith A, Tyrrell D, Coyle K, Higgins P, Willman J.Diurnal variation in the symptoms of colds and influenza. Chronobiol Int 1988;5(4):411-6.
62. Cohen HA, Neuman I, Nahum H. Blocking effect of vitamin C in exercise-induced asthma. Arch Pediatr Adolesc Med 1997;151(4):367-70.
63. Cohen S, Tyrrell Da, Smith AP. Psychological stress and susceptibility to the common cold.N Engl J Med 1991;325(9): 606-12.
64. Hemila H. Vitamin C supplementation and common cold symptoms: factors affecting the magnitude of the benefit. Med Hypotheses 1999;52(2):171-8.
65. Kongerud J, Crissman K, Hatch G, Alexis N.Ascorbic acid is decreased in induced sputum of mild asthmatics. Inhal Toxicol 2003;15(2):101-9.
66. Cohen HA, Neuman I, Nahum H. Blocking effect of vitamin C in exercise-induced asthma. Arch Pediatr Adolesc Med 1997;151(4):367-70.
67. Butland BK, Fehily AM, Elwood PC. Diet, lung function, and lung function decline in a cohort of 2512 middle aged men. Thorax 2000;55:102-8.
68. Hu G, Zhang X, Chen J, Peto R, Campbell TC, Cassano PA. Dietary vitamin C intake and lung function in rural China. Am J Epidemiol 1998;148:594-9.
69. Smit HA, Grievink L, Tabak C. Dietary influences on chronic obstructive lung disease and asthma: a review of the epidemiological evidence. Proc Nutr Soc 1999;58(2):309-19.
70. Gale CR, Martyn CN, Winter PD, Cooper C. Vitamin C and risk of death from stroke and coronary heart disease in cohort of elderly people. BMJ 1995;310:1563-6.
71. Bulpitt CJ. Vitamin C and vascular disease. BMJ 1995;310:1548-9.
72. Bordia AK.The effect of vitamin C on blood lipids, fibrinolytic activity, platelet adhesiveness in patients with coronary artery disease. Atherosclerosis 1980;35(2):181-7.
73. Nilsson TK, Sundell IB, Hellsten G, Hallmans G. Reduced plasminogen activator inhibitor activity in high consumers of fruits, vegetables and root vegetables. J Intern Med 1990;227(4):267-71.
74. Khaw KT, Woodhouse P. Interrelation of vitamin C, infection, haemostatic factors, and cardiovascular disease. BMJ 1995;310(6994):1559-63.
75. Sullivan DR. Screening for cardiovascular disease with cholesterol. Clin Chim Acta 2002;315(1-2):49-60.
76. Cerna O, Ginter E. Blood lipids and vitamin-C status. Lancet 1978; 1(8072):1055–6.
77. Greco AM, LaRocca L. Correlation between chronic hypovitaminosis C in old age and plasma levels of cholesterol and triglycerides. Int J Vit Nutr Res 1982;23:129-36.
78. Itoh R, Yamada K, Oka J, Echizen H, Suyama Y, Murakami K. Serum ascorbic acid and HDL cholesterol in a healthy elderly Japanese population. Int J Vitam Nutr Res 1990;60(4):360-5.
79. Simon JA, Hudes ES. Relation of serum ascorbic acid to serum lipids and lipoproteins in US adults. J Am Coll Nutr 1998;17(3):250-5.
80. Jacques PF, Sulsky SI, Perrone GE, Jenner J, Schaefer EJ. Effect of vitamin C supplementation on lipoprotein cholesterol, apolipoprotein, and triglyceride concentrations. Ann Epidemiol 1995;5(1):52-9.
81. Harwood HJ Jr, Greene YJ, Stacpoole PW. Inhibition of human leukocyte 3-hydroxy-3-methylglutaryl coenzyme A reductase activity by ascorbic acid. An effect mediated by the free radical monodehydroascorbate. J Biol Chem 1986;261(16): 7127-35.
82. Greene YJ, Harwood HJ Jr, Stacpoole PW. Ascorbic acid regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and cholesterol synthesis in guinea pig liver. Bioch Biophys Acta 1985; 834(1):134-8.
83. Santillo M, Santangelo F, Belfiore A, Mastursi M, Mondola P. Effect of ascorbic acid administration on B and E apoproteins in rats fed a cholesterol enriched diet. Horm Metab Res 1993;25(3):156-9.
84. Knekt P, Reunanen A, Jarvinen R, Seppanen R, Heliovaara M, Aromaa A. Antioxidant vitamin intake and coronary mortality in a longitudinal population study. Am J Epidemiol 1994; 139(12): 1180-9.
85. Stringer MD, Gorog PG, Freeman A, Kakkar VV. Lipid peroxides and atherosclerosis. BMJ 1989;298:281-4.
86. Diaz MN, Frei B, Vita JA, Keaney JF. Antioxidants and atherosclerotic heart disease. N Engl J Med 1997;337:408-16.
87. Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci U S A 1989;86(16):6377-81.
88. Abuja PM. Ascorbate prevents prooxidant effects of urate in oxidation of human low density lipoprotein. FEBS Lett 1999;446(2-3):305-8.
89. Lehr HA, Frei B, Olofsson AM, Carew TE, Arfors KE. Protection from oxidized LDL-induced leukocyte adhesion to microvascular and macrovascular endothelium in vivo by vitamin C but not by vitamin E. Circulation 1995;91(5):1525-32.
90. Mak S, Egri Z, Tanna G, Colman R, Newton GE. Vitamin C prevents hyperoxia-mediated vasoconstriction and impairment of endothelium-dependent vasodilation. Am J Physiol Heart Circ Physiol 2002;282(6):H2414-21.
91. Bayerle-Eder M, Pleiner J, Mittermayer F, Schaller G, Roden M, Waldhausl W, et al. Effect of systemic vitamin C on free fatty acid-induced lipid peroxidation. Diabetes Metab 2004;30(5):433-9.
92. Nakazono K, Watanabe N, Matsuno K, Sasaki J, Sato T, Inoue M. Does superoxide underlie the pathogenesis of hypertension? Proc Natl Acad Sci USA 1991;88:10045-8.
93. Moran JP, Cohen L, Greene JM, Xu G, Feldman EB, Hames CG, et al. Plasma ascorbic acid concentrations relate inversely to blood pressure in human subjects. Am J Clin Nutr 1993;57:213-7.
94. Bates CJ, Walmsley CM, Prentice A, Finch S. Does vitamin C reduce blood pressure? Results of a large study of people aged 65 or older. J Hyperten 1998;16(7):925-32.
95. Duffy SJ, Gokce N, Holbrook M, Huang A, Frei B, Keaney JF Jr, et al. Treatment of hypertension with ascorbic acid. Lancet 1999;354(9195):2048-9.
96. Chen J, He J, Hamm L, Batuman V, Whelton PK. Serum antioxidant vitamins and blood pressure in the United States population. Hypertension 2002;40(6):810-6.
97. Kritchevsky SB, Shimakawa T, Tell GS, Dennis B, Carpenter M, Eckfeldt JH, et al. Dietary antioxidants and carotid artery wall thickness. The ARIC Study. Atherosclerosis risk in communities study. Circulation 1995;92:2142 –50.
98. Joshipura KJ, Hu FB, Manson JE, Stampfer MJ, Rimm EB, Speizer FE, et al. The effect of fruit and vegetable intake on risk for coronary heart disease. Ann Intern Med 2001;134:1106-14.
99. Zandi PP, Anthony JC, Khachaturian AS, Stone SV, Gustafson D, Tschanz JT, et al. Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: the Cache County Study. Arch Neurol 2004;61(1):82-8.
100. Montilla-Lopez P, Munoz-Agueda MC, Feijoo Lopez M, Munoz-Castaneda JR, Bujalance-Arenas I, et al. Comparison of melatonin versus vitamin C on oxidative stress and antioxidant enzyme activity in Alzheimer’s disease induced by okadaic acid in neuroblastoma cells. Eur J Pharmacol 2002;451(3):237-43.
101. Parle M, Dhingra D. Ascorbic acid: a promising memory-enhancer in mice. J Pharmacol Sci 2003;93(2):129-35.
102. Chan SW, Reade PC. The role of ascorbicacid in oral cancer and carcinogenesis. Oral Dis 1998;4(2):120-9.
103. Lupulescu A. The role of vitamins A, beta-carotene, E and C in cancer cell biology. Int J Vitam Nutr Res 1994;64(1):3-14.
104. Murata A, Morishige F, Yamaguchi H. Prolongation of survival times of terminal cancer patients by administration of large doses of ascorbate. Int J Vitam Nutr Res 1982;23:103-13.
105. Moertel CG, Fleming TR, Creagan ET, Rubin J, O’connell MJ, Ames MM. High-dose vitamin C versus placebo in the treatment of patients with advanced cancer who have had no prior chemotherapy. A randomized double-blind comparison. N Engl J Med 1985;312(3):137-44.
106. Halliwell B. Vitamin C and genomic stability. Mutat Res 2001;475(1-2):29-35.
107. Moorman PG, Ricciuti MF, Millikan RC, Newman B. Vitamin supplement use and breast cancer in a North Carolina population. Public Health Nutr 2001;4(3):821-7.
108. Block G. Epidemiologic evidence regarding vitamin C and cancer. Am J Clin Nutr 1991;54(6):S1310-4.
109. Dusinska M, Kazimirova A, Barancokova M, Beno M, Smolkova B, Horska A, et al. Nutritional supplementation with antioxidants decreases chromosomal damage in humans. Mutagenesis 2003;18(4):371-6.
110. Koh WS, Lee SJ, Lee H, Park C, Park MH, Kim WS, et al. Differential effects and transport kinetics of ascorbate derivatives in leukemic cell lines. Anticancer Res 1998;18:2487-93.
111. Padayatty SJ, Sun H, Wang Y, Riordan HD, Hewitt SM, Katz A, et al. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med 2004;140(7):533-7.
112. Graumlich JF, Ludden TM, Conry-Cantilena C, Cantilena LR Jr, Wang Y, Levine M. Pharmacokinetic model of ascorbic acid in healthy male volunteers during depletion and repletion. Pharm Res 1997;14:1133-9.
113. Padayatty SJ, Riordan HD, Hewitt SM, Katz A, Hoffer LJ, Levine M. Intravenously administered vitamin C as cancer therapy: three cases. CMAJ 2006;174(7):937-42.
114. Chen Q, Espey MG, Krishna MC, Mitchell JB, Corpe CP, Buettner GR, et al. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl Acad Sci U S A 2005;102(38):13604-9.
115. Byers T, Guerrero N. Epidemiologic evidence for vitamin C and vitamin E in cancer prevention. Am J Clin Nutr 1995;62:1385S -92S.
116. Jenab M, Riboli E, Ferrari P, Sabate J, Slimani N, Norat T, et al. Plasma and dietary vitamin C levels and risk of gastric cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST). Carcinogenesis 2006 Jun 14; Epub ahead of print.
117. Sakagami H, Satoh K, Hakeda Y, Kumegawa M. Apoptosis-inducing activity of vitamin C and vitamin K. Cell Mol Biol 2000;46:129-143.
118. Kang JS, Cho D, Kim YI, Hahm E, Yang Y, Kim D, et al. L-ascorbic acid (vitamin C) induces the apoptosis of B16 murine melanoma cells via a caspase-8-independent pathway. Cancer Immunol Immunother 2003;52(11):693-698.
119. Thomas CG, Vezyraki PE, Kalfakakou VP, Evangelou AM. Vitamin C transiently arrests cancer cell cycle progression in S phase and G(2)/M boundary by modulating the kinetics of activation and the subcellular localization of Cdc25C phosphatase. J Cell Physiol 2005;205(2):310-8.
120. Naidu KA, Tang JL, Naidu KA, Prockop LD, Nicosia SV, Coppola D. Antiproliferative and apoptotic effect of ascorbyl stearate in human glioblastoma multiforme cell: Modulation of insulin-like growth factor-I receptor (IGF-IR) expression. J Neurooncol 2001;54(1):15-22.
121. Naidu AK, Karl RC, Naidu KA, Coppola D. The antiproliferative and pro-apoptotic effect of Ascorbyl Stearate in Human pancreatic cancer cells: Association with decreased expression of insulin-like growth factor receptor-1. Digest Dis Sci 2003;48(1):230-7.
122. Naidu AK. Vitamin C in human health and disease is still a mystery? An overview. Nutr J 2003;2(1):7.
123. Bonnefont-Rousselot D. The role of antioxidant micronutrients in the prevention of diabetic complications. Treat Endocrinol 2004;3(1):41-52.
124. Chen L, Jia RH, Qiu CJ, Ding G. Hyperglycemia inhibits the uptake of dehydroascorbate in tubular epithelial cell. Am J Nephrol 2005;25(5):459-65.
125. Timimi FK, Ting HH, Haley EA, Roddy MA, Ganz P, Creager MA. Vitamin C improves endothelium-dependent vasodilation in patients with insulin-dependent diabetes mellitus. J Am Coll Cardiol 1998;31(3):552-7.
126. Ting HH, Timimi FK, Boles KS, Creager SJ, Ganz P, Creager MA. Vitamin C improves endothelium-dependent vasodilation in patients with non-insulin-dependent diabetes mellitus. J Clin Invest 1996;97(1):22-8.
127. Chen H, Karne RJ, Hall G, Campia U, Panza JA, Cannon RO 3rd, Wang Y, Katz A, Levine M, Quon MJ. High dose oral vitamin C partially replenishes vitamin C levels in patients with type 2 diabetes and low vitamin C levels but does not improve endothelial dysfunction or insulin resistance. Am J Physiol Heart Circ Physiol 2006;290(1):H137-45.
128. Choi SW, Benzie IF, Lam CS, Chat SW, Lam J, Yiu CH, et al. Inter-relationships between DNA damage, ascorbic acid and glycaemic control in Type 2 diabetes mellitus. Diabet Med 2005;22(10):1347-53.
129. Simon JA, Hudes ES. Serum ascorbic acid and other correlates of self-reported cataract among older Americans. J Clin Epidemiol 1999;52(12):1207-11.
130. Van der Pols JC. A possible role for vitamin C in age-related cataract. Proc Nutr Soc 1999;58(2):295-301.
131. Cheng R, Lin B, Lee KW, Ortwerth BJ. Similarity of the yellow chromophores isolated from human cataracts with those from ascorbic acid-modified calf lens proteins: evidence for ascorbic acid glycation during cataract formation. Biochim Biophys Acta 2001;1537(1):14-26.
132. Argirov OK, Lin B, Ortwerth BJ. 2-ammonio-6-(3-oxidopyridinium-1-yl)hexanoate (OP-lysine) is a newly identified advanced glycation end product in cataractous and aged human lenses. J Biol Chem 2004;279(8):6487-95.
133. Jacques PF. The potential preventive effects of vitamins for cataract and age-related macular degeneration. Int J Vitam Nutr Res 1999;69(3):198-205.
134. Hathcock JN, Azzi A, Blumberg J, Bray T, Dickinson A, Frei B, et al. Vitamins E and C are safe across a broad range of intakes. Am J Clin Nutr 2005;81(4):736-45.
135. Rees DC, Kelsey H, Richards JD. Acute haemolysis induced by high dose ascorbic acid in glucose-6-phosphate dehydrogenase deficiency. BMJ 1993;306:841-2.
136. Hallberg L. Iron and vitamins. Bibl Nutr Dieta 1995;52:20-9.
137. Cook JD, Watson SS, Simpson KM, Lipschitz DA, Skikne BS. The effect of high ascorbic acid supplementation on body iron stores. Blood 1984;64:721-6.
138. Diplock AT. Antioxidant nutrients and disease prevention: An overview. Am J Clin Nutr 1991;53(1):S189- 93.
139. Meyers DG, Maloley PA, Weeks D. Safety of antioxidant vitamins. Arch Intern Med 1996;156:925-35.
140. Gerster H. No contribution of ascorbic acid to renal calcium oxalate stones. Ann Nutr Metab 1997;41(5):269-82.
141. Wandzilak TR, D’Andre SD, Davis PA, Williams HE. Effect of high dose vitamin C on urinary oxalate levels. J Urol 1994;151(4):834-37.
142. Curhan GC, Willett WC, Rimm EB, Stampfer MJ. A prospective study of the intake of vitamins C, B6, and the risk of kidney stones in men. J Urol 1996;155:1847-51.
143. Auer BL, Auer D, Rodgers AL. Relative hyperoxaluria, crystalluria and haematuria after megadose ingestion of vitamin C.Eur J Clin Invest 1998;28(9):695-700.
144. Pena de la Vega L, Lieske JC, Milliner D, Gonyea J, Kelly DG. Urinary oxalate excretion increases in home parenteral nutrition patients on a higher intravenous ascorbic acid dose. J Parenter Enteral Nutr 2004;28(6):435-8.
145. Rivers JM. Safety of high-level vitamin C ingestion. Int J Vitam Nutr Res 1989;30:95-102.
146. Stein HB, Hasan A, Fox IH. Ascorbic acid-induced uricosuria. Ann Intern Med 1976;84:385-8.
147. Schmidt KH, Hagmaier V, Hornig DH, Vuilleumier JP, Rutishauser G. Urinary oxalate excretion after large intakes of ascorbic acid in man. Am J Clin Nutr 1981;34(3):305-11.
148. Mitch WE, Johnson MW, Kirshenbaum JM, Lopez RE. Effect of large oral doses of ascorbic acid on uric acid excretion by normal subjects. Clin Pharmacol Ther 1981;29:318-21.
149. Wynne H, Khan T, Avery P, Wood P, Ward A, Kamali F. Dietary related plasma vitamin C concentration has no effect on anticoagulation response to warfarin. Thromb Res 2005 Aug 30; Epub ahead of print.
150. Slain D, Amsden JR, Khakoo RA, Fisher MA, Lalka D, Hobbs GR. Effect of high-dose vitamin C on the steady-state pharmacokinetics of the protease inhibitor indinavir in healthy volunteers. Pharmacotherapy 2005;25(2):165-70.
151. Nagayama H, Hamamoto M, Ueda M, Nito C, Yamaguchi H, Katayama Y. The effect of ascorbic acid on the pharmacokinetics of levodopa in elderly patients with Parkinson disease. Clin Neuropharmacol 2004;27(6):270-3.