Introduction
Osteoarthritis, also called degenerative joint disease is the most common of all joint diseases (1). Primary or secondary osteoarthritis constitutes a substantial problem in theelderly, and is more prevalent in women than in men. Etiopathogenesis of primary osteoarthritis is not clear. Loosing the metabolic balance between the synthesis and degradation of the cartilage and subchondral bone leads to predominance of catabolic over anabolic processes that resultsin progressive destruction of joint tissues (2). The repair process is impaired or may lead to overgrowth of joint structures (3,4). In the course of osteoarthritis, subchondral bone and cartilage may have different histological structure and biomechanical properties (5). Recently, it has been suggested that unequal mineralization, that occurs in the subchondral bone, and increased bone turnover could lead to degeneration and loss of the cartilage (6). Also, subchondral osteonecrosis may cause changes in cartilage structure (7,8). The pathogenesis of osteoarthritis involves altered interactions of different types of chondrocytes and constituents of extracellular matrix such as growth factors, interleukins and specific enzymes (9). Some growth factors like insulin-like growth factor-1 (IGF-1) and transforming growth factor-β (TGF-β) play an essential anabolic role in cartilage and bone metabolism. IGF-1 stimulates proteoglycan and collagen synthesis but its action in osteoarthritis on cartilage and bone cells could be modified (6,10,11). Impaired balance between IGF-1 and specific binding proteins (IGFBPs), inhibitory effect of cytokines and mechanical factors take part in the development of the disease (12). In addition, chondrocytes in osteoarthritis are less sensitive to IGF-1 that may be caused by decreased expression of surface IGF-1 receptors or enhanced activity of cytokines (13,14,15). The contribution of each mechanism in the course of osteoarthritis depends on its stage since the disease progression is accompanied by changes in chondrocytes presenting different metabolic activities (9,16).
The aim of our study was to compare the concentrations of biochemical markers such as IGF-1, bone turnover markers and cytokines in elderly patients with advanced primary hip osteoarthritis or aseptic osteonecrosis of the hip and to evaluate associations between these analytes in serum and synovial fluid in relation to disease etiopathogenesis.
Materials and methods
Samples of joint fluid and blood were collected from 23 patients who underwent hip surgery in years 2003-2004 in the Department of Orthopedics and Traumatology at University Hospital in Bydgoszcz. 17 hips (13 women and 4 men, 68±9 years old) were operated on because of primary osteoarthritis (POA group), and 6 hips (5 women and 1 man, 68±15 years old) because of osteonecrosis (SOA group), all classified on the basis of clinical features and radiographic changes. Blood was collected during the week preceding the surgery and serum samples were kept frozen at -70 °C until used. Samples of synovial fluid were collected with a syringe before incising the capsule, centrifuged, treated with hyaluronidase as we previously described and stored at -70 °C for up to 1 month before the analyses (17).
Informed consent was given by all participants and the procedures were approved by the local Bioethics Committee.
All specimens were assayed for IGF-1, CTx (ß-crosslaps), BSAP (bone alkaline phosphatase) and cytokines: IL-6, IL-8 and IL-10 using commercially available ELISA kits. CTx, a bone resorption marker was assayed by Serum CrossLaps (Osteometer BioTech, Denmark), the activity of BSAP, a bone formation marker, by ALKPHASE-B (Metra Biosystems, USA), IGF-1 by Octeia (IGF-1, IDS, UK) and IL-6, IL-8, IL-10 concentrations by Bender Medsystems, Austria.
Statistical analysis
Statistical analysis was done using the t-Student test and Pearson correlation coefficient. P-value < 0.05 was considered statistically significant.
Only positive predictive value (PPV) was calculated as one of the measures of diagnostic accuracy.
Results
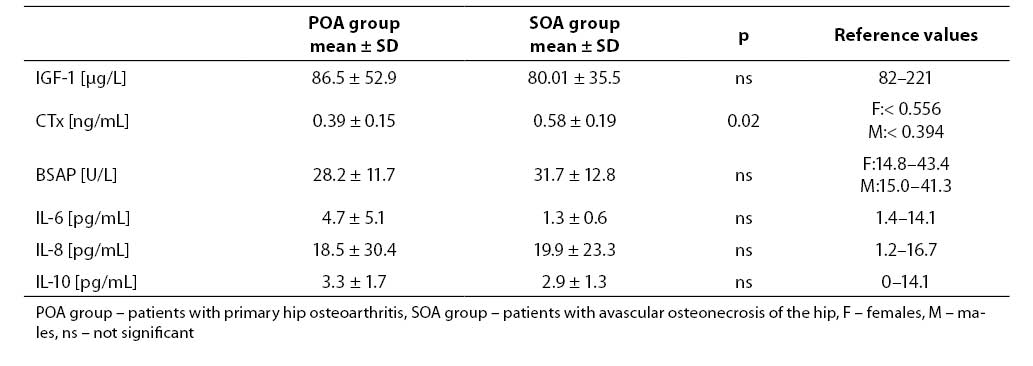
Patients with avascular osteonecrosis of the hip had very low serum IGF-1 level (Table 1). Mean serum concentration of IGF-1 in patients with primary hip osteoarthritis was within the normal range for adults. In patients with osteonecrosis, the mean level of IGF-1 in serum was lower than 82 mg/l. Positive predictive value of serum IGF-1 in cases with primary hip osteoarthritis was 71% while in cases with avascular osteonecrosis of the hip was 83%. In our study, serum IGF-1 below 82 mg/l was accepted as true positive result in patients with primary and secondary hip osteoarthritis.
Mean serum concentration or activity of all measured parameters, except CTx, did not differ significantly in both groups.
We found higher concentration of serum CTx in cases with avascular osteonecrosis of the hip and only in this group mean serum CTx value was abovethe upper reference range for healthy adults. The activity of serum BSAP was within the normal range in patients from both groups (Table 1).
Table 1. Mean values and reference values of analytes measured in the serum.
Serum levels of individual cytokines were similar in both groups. Mean serum IL-6 concentration was low only in osteonecrosis patients while IL-8 level was increased in both groups. Mean serum IL-10 was within the reference range.
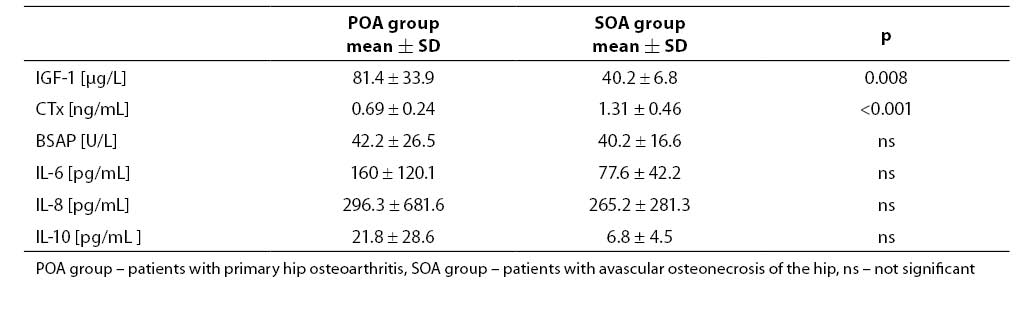
Mean synovial fluid IGF-1 value in cases with avascular osteonecrosis of the hip was significantly lower and CTx was higher than in those with primary hip osteoarthritis (Table 2).Activity of BSAP was similar in both groups. There wasa tendency to lower cytokine values, especially for IL-6 and IL-10, in the synovial fluid of patients with avascular osteonecrosis of the hip compared to those with primary hip osteoarthritis; however, more pronounced proinflammatory status was found in osteonecrosis. The quotients IL-6: IL-10 and IL-8: IL-10 were up to 3-fold higher in patients with osteonecrosis.
Table 2. Mean values of parameters measured in the synovial fluid.
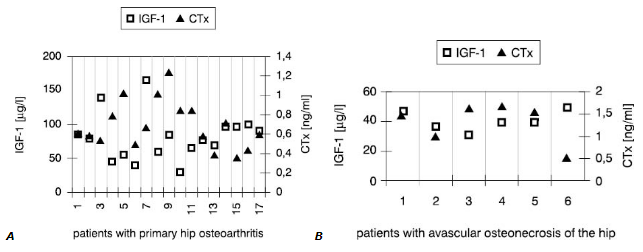
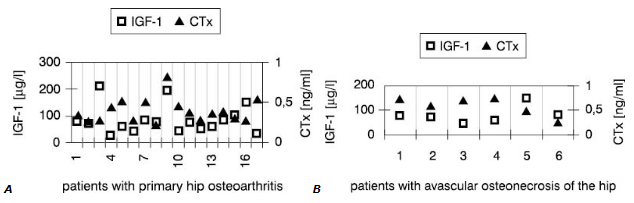
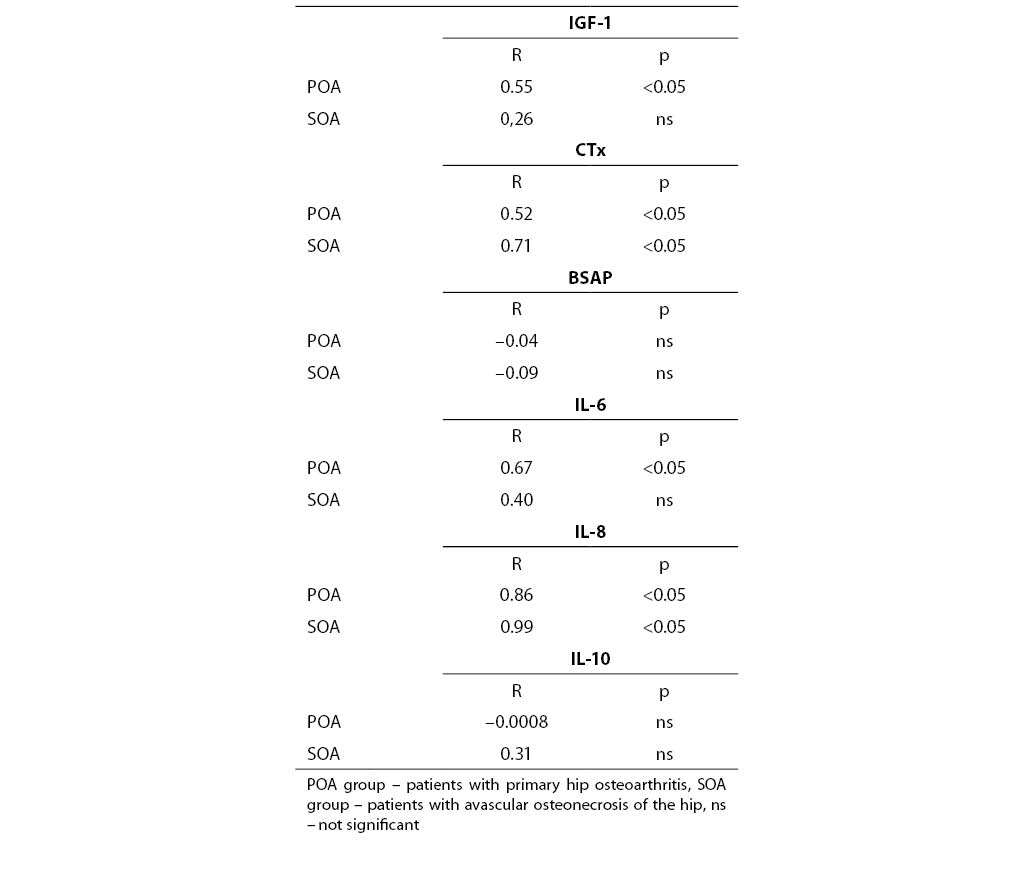
Interestingly, synovial fluid IGF-1 concentrations (Fig.1) in all patients but one were low<er compared with these in serum (Fig.2). However, only in SOA cases this difference reached statistical significance (Table 3).
Figure 1. Individual values of IGF-1 and CTx in the synovial fluid
Figure 2. Individual values of IGF-1 and CTx in the serum
Table 3. Correlations between biochemical markers measured in serum and synovial fluid.
On the contrary, values of other markers measured in the joint fluid were much higher than in serum in both groups. This was primarilyobserved when concentrations of cytokines in synovial fluid and serum were compared. In most cases, higher CTx in synovial fluid accompanied low IGF-1 concentration.
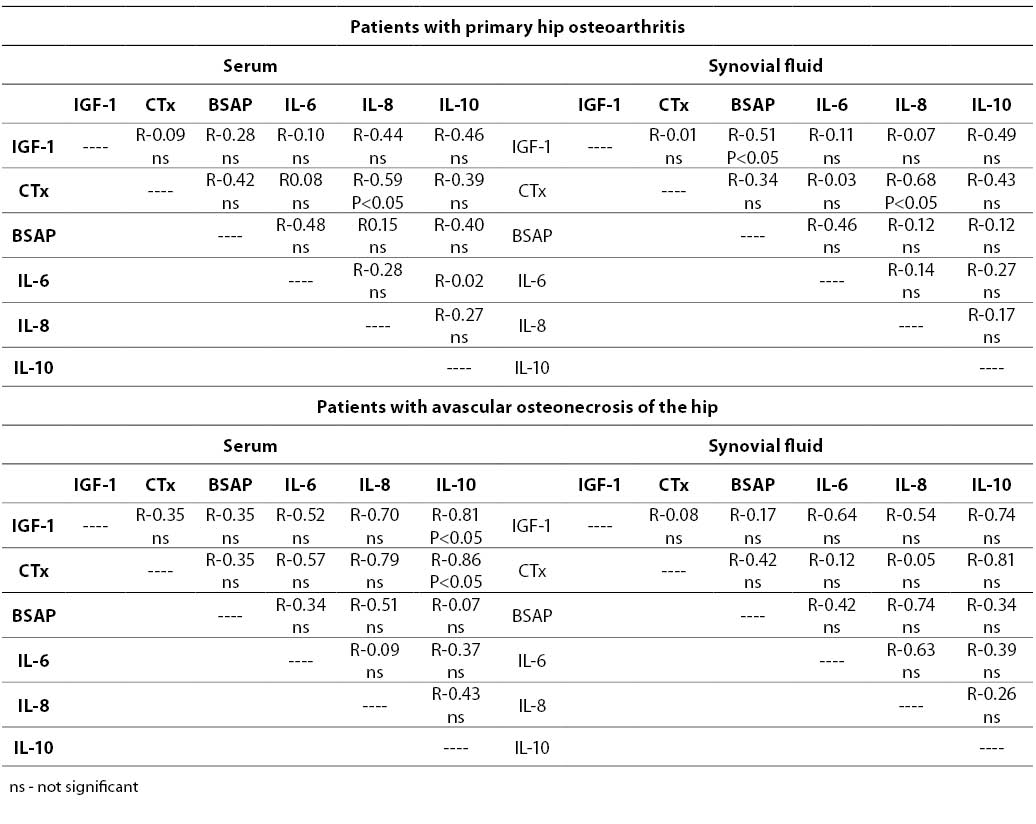
In patients with primary hip osteoarthritis IGF-1, CTx, IL-6 and IL-8 concentrations measured in the synovial fluid correlated with those in the serum (Table 3). Similar and strong associations, but only for CTx and IL-8, were noted in the SOA group. There was no correlation between BSAP activity in the serum and synovial fluid.
The associations between various parameters analyzed in this study are shown in Table 4. Good positive correlation in POA patients was noted, primarily between CTx and IL-8.
In the SOA group, serum IGF-1 correlated with IL-10, and a strong negative association was found between serum CTx and IL-10 (Table 4).
Table 4. Associations between biochemical markers in both groups of patients
Discussion
IGF-1 deficiency is regarded as an essential contributor to degenerative changes in the cartilage (18,19,20,21). Previous reports showed that local (synovial fluid) IGF-1 concentration is low in osteoarthritis (22,23,24,25). Hedstrom et al. reported reduced serum IGF-1 levels in hip fracture patients compared with hip osteoarthritis (26). Recently it was shown that IGF-1 concentrations in the synovial fluid and serum in patients with aseptic prosthesis loosening were lower than in osteoarthritis (27)..We found that both serum and synovial fluid concentrations of IGF-1 were low in elderly patients who underwent hip surgery because of hip osteoarthritis. IGF-1 level was in most cases in both groups below the reference range (PPV 71% or 83%). Similarly, Pagura et al. observed lower concentration of this growth factor in serum and synovial fluid of females awaiting knee arthroplasty than in controls (21). Contrary to this, the levels of other assayed parameters were much higher locally than in the serum. This observation confirms the data we reported earlier (17,28,29).
In their study, Denko et al. found much lower IGF-1 concentration in the synovial fluid than in the serum of patients with osteoarthritis and different forms of rheumatoid arthritis (25). We suggest that the pathogenesis of osteoarthritis influences the local concentration of cartilage degradation and bone turnover indices within the joint. Mean synovial fluid concentration of an anabolic factor, such as IGF-1, was found to be significantly lower in cases with avascular osteonecrosis of the hip.
Hilal et al. reported increased response of osteoblast-like cells after stimulation with IGF-1 and other growth factors, resulting in augmented expression of alkaline phosphatase (30). Despite the observed direct association of IGF-1 with BSAP and low concentration of IGF-1, especially in the synovial fluid of patients with avascular osteonecrosis of the hip, normal activity of BSAP was detected.
According to Matsumoto et al., serum IGF-1 is decreased in osteoarthritis with bone erosions (19). We found that low IGF-1 concentrations were accompanied by higher values of CTx. However, enhanced bone resorption, indicating increased bone collagen degradation, was demonstrated only in patients with avascular osteonecrosis. Lajeunesse et al. suggested that imbalance between bone resorption and formation during remodeling may be responsible for abnormal metabolism of subchondral bone in osteoarthritis (31). Indeed, in our study bone resorption, reflected by CTx, was not correlated with bone formation, measured as activity of BSAP, even in patients with avascular osteonecrosis of the hip.
It has been reported that the levels of proinflammatory interleukins, especially IL-8 in synovial fluid, may be increased in conditions associated with bone loss such as osteoarthritis and rheumatoid arthritis (32). Despite similar values of IL-8 found in both groups of patients, 3-fold higher ratio of IL-8 compared to anti-inflammatory IL-10 in patients with osteonecrosis may suggest a proinflammatory status in this patient group. The data reported here indicate a possibility that avascular osteonrecrosis of the hip may be characterized by a more catabolic state reflected by low IGF-1 with concomitant increase in bone resorption and proinflammatory indices.
This study suffers from some limitations such as a small number of cases with hip osteoarthritis and, especially, cases with avascular osteonecrosis. However, it should be taken into consideration that the availability of synovial fluid as a biological material for laboratory assays is often very limited.
IGF-1 level may constitute a risk factor predisposing to osteoarthritis and seems to be related to etiopathogenesis of the disease. Very low IGF-1 concentration in serum or locally in synovial fluid may indicate osteonecrosis.
Acknowledgments
This study was initiated by Prof. PJ. Bilinski MD, PhD, Head of theDepartment of Orthopedics and Traumatology. This study was supported by Collegium Medicum, Nicolaus Copernicus University in Bydgoszcz, grant BW 14/2002.
References
1. Brandt KD. Internal Medicine. Osteoarthritis. Fourth Edition. Mosby-Year Book, Inc. 1994:2489
2. Mollenhauer JA, Erdmann S. Introduction: molecular and biomechanical basis of osteoarthritis. Cell Mol Life Sci 2002;59:3-4.
3. Saris DBF, Dhert WJA, Verbout AJ. Joint homeostasis: The discrepancy between old and fresh defects in cartilage repair. J Bone Joint Surg 2003;85-B:1067-76.
4. Henrotin Y, Reginster JY. Anabolic events in osteoarthritis. Osteoarthritis Cartilage 1999;7:310-2.
5. Hunter DJ, Spector TD. The role of bone metabolism in osteoarthritis. Curr Rheumatology Rep 2003;5:15-9.
6. Lajeunesse D. Alterated subchondral osteoblast cellular metabolism in osteoarthritis: cytokines, eicosanoids, and growth factors. J Musculoskel Neuron Interac 2002;2:504-6.
7. Yamamoto T, Yamaguchi T, Lee KB, Bullough PG. A clinicopathologic study of osteonecrosis in osteoarthritic hip. Osteoarthritis Cartilage 2000;8:303-8.
8. Pavelka K. Osteonecrosis. Bailliere’s Clin Rheumatology 2000;14: 399-414.
9. Tesche F, Miosge N. New aspects of the pathogenesis of osteoarthritis: the role of fibroblast-like chondrocytes in late stages of the disease. Histol Histopathol 2005;20:329-37.
10. Goldberg A. Effects of growth factors on articular cartilage. Ortopedia Traumatologia Rehabilitacja 2001;3:190-3.
11. Martel-Pelletier J, Di Batista JA, Lajeunesse D, Pelletier JP. IGF/IGFBP axis in cartilage and bone in osteoarthritis pathogenesis. Inflamm Res 1998;47:90-100.
12. Trippel SB. Growth factor inhibition: potential role in the etiopathogenesisof osteoarthritis. Clin Orthop Relat Res 2004;Suppl 427:47-52.
13. Malemud CJ. Cytokines as therapeutic targets for osteoarthritis. BioDrugs 2004;18:23-35.
14. Denko CW, Malemud CJ. Role of growth hormone/insulin growth factor-1 paracrine axis in rheumatic diseases. Semin Arthritis Rheumat 2005;35:24-34.
15. Wang J, Verdonk P, Elewaut D, Veys EM, Verbruggen G. Homeostasis of the extracellular matrix of normal and osteoarthritic human articular cartilage chondrocytes in vitro. Osteoarthritis Cartilage 2003;11:801-9.
16. Aigner T, McKenna L. Molecular pathology and pathobiology of osteoarthriticcartilage. Cell Mol Life Sci 2002;59:5-18.
17. Sypniewska G, Lis K, Bilinski PJ. Bone turnover markers and cytokines in joint fluid: analyses in 10 patients with loose hip prosthesis and 39 with hip osteoarthritis. Acta Orthop Scand 2002;73:518-22.
18. McAlindon TE, Teale JD, Dieppe PA. Levels of insulin related growth factor 1 in osteoarthritisof the knee. Ann Rheum Dis 1993;52:229-31.
19. Matsumoto T, Tsurumoto T. Serum YKL-40 levels in rheumatoid arthritis: correlations between clinical and laboratory parameters. Clin Exp Rheumatol 2001;19:655-60.
20. Pagura SM, Thomas SG, Woodhouse LJ, Ezzat S. Women awaiting knee replacement have reduced function of growth hormone. Clin Orthop Relat Res 2003; 415:202-13.
21. Pagura SM, Thomas SG, Woodhouse LJ, Ezzat S, Marks P. Circulating and synovial fluid levels of IGF-1, cytokines, physical function and anthropometry differ in women awaiting total knee arthroplasty when compared to men. J Orthop Res 2005;23:397-405.
22. Tavera C, Abribat T, Reboul P, Dore S, Brazeau P, Pelletier JP, Martel-Pelletier J. IGF and IGF-binding protein system in the synovial fluid of osteoarthritic and rheumatoid arthritic patients. Osteoarthritis Cartilage 1996;4:263-74.
23. Fernihough JK, Billingham ME, Cwyfan-Huges S, Holly JM. Local disruption of insulin-like growth factor system in the arthritic joint. Arthritis Rheum 1996;39:1556-65.
24. Whellams EJ, Maile LA, Ferninhough JK, Billingham ME, Holly JM. Alterations in insulin-like growth factor binding protein-3 proteolysis and complex formation in the arthritic joint. J Endocrinology 2000;165: 545-56.
25. Denko CW, Boja B, Moskowitz RW. Growth factors, insulin-like growth factor-1 and growth hormone, in synovial fluid and serum of patients with rheumatic disorders. Osteoarthritis Cartilage 1996;4:245-9.
26. Hedstrom M, Saaf M, Dalen N. Low IGF-1 levels in hip fracture patients. A comparison of 20 coxarthrotic and 23 hip fracture patients. Acta Orthop Scand 1999;70:145-8.
27. Andersson MK, Stark A, Anissian L, Mohan S, Tsai JA. Low IGF-1 in synovial fluid and serum in patients with aseptic prosthesis loosening. Acta Orthop 2005;76:320-5.
28. Lis K, Sypniewska G, Bilinski PJ. Interleukin-6, insulin-like growth factor 1 and markers of bone and cartilage destruction in serum and synovial fluid. (abstract) Eur J Clin Invest 2002;32(S2):15.
29. Lis K, Odrowaz-Sypniewska G, Bilinski PJ, Mątewski D, Krieger I. Cartilage Oligomeric Matrix Protein (COMP) and glycoprotein YKL-40 as markers of cartilage degradation and inflammation in osteoarthritis. Diagn Lab 2003;39:133-41.
30. Hilal G, Martel-Pelletier J, Pelletier JP, Ranger P, Lajeunesse D. Osteoblast-like cells from human subchondral osteoarthritic bone demonstrate an altered phenotype in vitro: possible role in subchondral bone sclerosis. Arthritis Rheuma 1998;41:891-9.
31. Lajeunesse D, Reboul P. Subchondral bone in osteoarthritis: a biologic link with articular cartilage leading to abnormal remodeling. Curr Opinion Rheumatology 2003;15:628-33.
32. Kaneko S, Satoh T, Chiba J, Ju C, Inoue K, Kagawa J. Interleukin-6 and interleukin-8 levels in serum and synovial fluid of patients with osteoarthritis. Cytokines Cell Mol Ther 2000;6:71-9.