C-reactive protein in children with asthma and allergic rhinitis
Daniela Galez
[*]
[1]
Slavica Dodig
[1]
Miljenko Raos
[1]
Boro Nogalo
[1]
Introduction
Allergic respiratory diseases, which are among the most common chronic diseases in children (1,2), may manifest with symptoms in the upper (allergic rhinitis and sinusitis) and lower (allergic asthma) airways. As the histology, allergic disease epidemiology, mechanisms of inflammation, triggers for allergic disease clinical manifestations, diagnostic procedures and treatment are common to both upper and lower airways, they can be referred to as an integral respiratory system (3). Accordingly, asthma and rhinitis can be considered as manifestations of a single chronic allergic respiratory syndrome (4).
Chronic airway inflammation as one of the major features of asthma and allergic rhinitis involves many cell types, of which mastocytes, eosinophilic granulocytes and T-lymphocytes play most important roles. In sensitive individuals, the inflammation induced by environmental allergens leads to the symptoms of asthma (bronchoconstriction, cough and chest tightness, frequently overnight or at dawn) (5,6) and rhinitis (nasal congestion, rhinorrhea, sneezing, nose itching) (7). The discomforts are usually reversible and resolve spontaneously or with therapy.
C-reactive protein (CRP) is a well known inflammation marker. Serum concentration of CRP is generally determined to assess a systemic inflammation (8), e.g., pneumonia, rheumatic disease, intestinal disease, etc. (9,10). It has recently been observed that CRP, even in the reference interval, i.e. determination of high sensitive CRP (hsCRP), can serve as a relevant prognostic marker in patients with cardiovascular disease (11) or diabetes mellitus (12). Determination of hsCRP concentration implies determination of CRP concentration by the established turbidimetric method on latex particles but adjusted to the low measurement area. So, hsCRP can also be used to assess the grade of inflammation in asthma patients (13).
As inflammation is one of the major characteristics of respiratory allergic diseases, the aim of this study was to estimate whether determination of CRP concentration would be of use as a marker of inflammation in children with asthma and allergic rhinitis.
Materials and methods
Subjects
The study included 42 healthy children (control group), mean (x±SD) age 9±5 years, and 70 pediatric patients during regular control of respiratory diseases, asthma (n=47) and rhinitis (n=23), mean (x±SD) age 7±4 years. Control group consisted of clinically healthy children without information of atopic status referred for systematic medical check-up at Srebrnjak Children's Hospital. The diagnosis of allergic disease was based on clinical criteria (personal and family history, physical examination, pulmonary function measurement, provocation skin tests) and laboratory testing (increased concentration of total and specific IgE antibodies, blood and nasal swab eosinophilic granulocyte count). The study groups included children without diabetes mellitus. Body mass index was uniform in both study groups (between 5th and 85th centile values for age). Children with acute viral or bacterial infection of the airways were excluded. All patients were referred from primary health care offices to Srebrnjak Children’s Hospital in Zagreb between January and June 2006. Diagnostic work-up was performed according to standardized procedure, and in line with ethical principles (approved by the Hospital Ethics Board) and Declaration on Human Rights from Helsinki 1975 and Tokyo amendments 2004 (14). Blood sampling was done upon clinical examination at outpatient clinics of allergology and pulmonology, between 8.00 a.m. and 3.00 p.m.
Methods
CRP concentration was determined by immunoturbidimetric method on latex particles (15), on an Olympus AU 400 biochemistry analyzer, using reagents from the same manufacturer. CRP concentration was determined in two ways: (a) a method with linearity of 0.2 to 480 mg/L, and (b) a method in low measurement area (linearity of 0.08 to160 mg/L; hsCRP). The concentrations of complement components C3 and C4 and of alpha1-antitrypsin (AAT) were determined by immunoturbidimetric method on an Olympus AU 400 biochemistry analyzer, using reagents from the same manufacturer. Leukocyte and platelet counts were measured on a Sysmex XT-1800i blood counter.
Statistics
Data storage and processing for statistical analysis were performed by use of Excel 2000 Microsoft Office software (Microsoft, USA). Data with normal distribution were described by arithmetic mean (χ) and standard deviation (SD), and data with asymmetric distribution were described by median (M) and interval. Data distribution was assessed by χ2-test, with p>0.05 as evidence of normal distribution. Statistical significance of between-group differences was assessed by ANOVA test (for normal distribution) or Kruskal-Wallis test (for asymmetric distribution); between-group relation was assessed by Student's t-test (for normal distribution) or Wilcoxon test (for asymmetric distribution). Statistical significance was set at p<0.05 (16). The MedCalc® statistical package, version 4.10-Windows 95, was employed (17).
Results
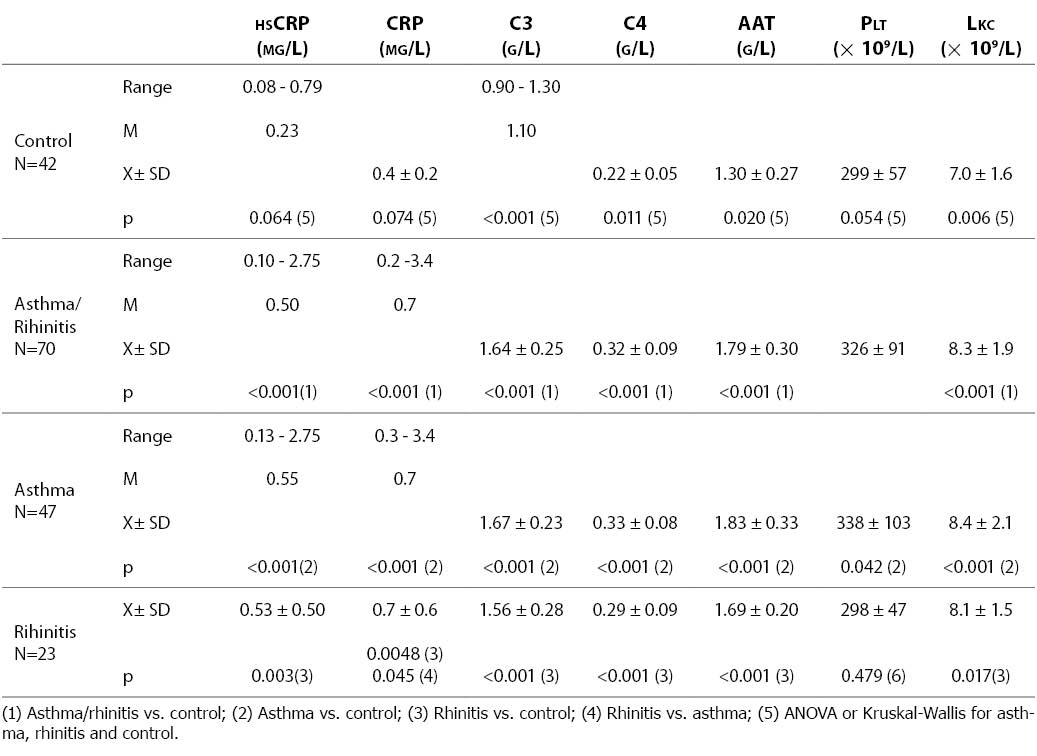
The group of children with allergic diseases were presented in two modes: in total, irrespective of diagnosis, and in subgroups according to diagnosis (asthma and allergic rhinitis), for statistical analysis to be performed for the group as a whole and for each subgroup in separate. Results obtained on the concentrations of hsCRP, CRP, C3, C4, AAT, leukocyte count and platelet count in the control group of healthy children and the group of children with respiratory allergic diseases (asthma and rhinitis) are presented in Table 1. As between-group differences were statistically significant for C3, C4, AAT and leukocyte count, and borderline for hsCRP, CRP and platelet count, between group analysis was performed. The concentration of CRP was statistically significantly higher in patients with asthma and rhinitis than in the control group, irrespective of the method of determination. The levels of C3, C4, AAT and leukocyte count were also statistically significantly higher in the patient group, either in total or in groups according to diagnosis. Platelet count was statistically significantly higher in asthma patients but not in rhinitis patients as compared with the control group of healthy children. The mean hsCRP concentration was statistically significantly higher in children with allergic diseases (0.65±0.55 mg/L) than in control group children (0.28±0.16 mg/L). The patients with asthma showed higher values of the upper range limit for hsCRP (2.75 mg/L) than patients with allergic rhinitis (1.57 mg/L). Only the concentration of CRP measured by the conventional procedure was statistically significantly lower in rhinitis patients as compared with asthma patients, whereas the values of other tests did not differ significantly between these two subgroups.
Table 1. HsCRP, CRP, C3, C4, A1-AT concentrations, and platelet and leukocyte counts in healthy subjects and patients with respiratory allergic diseases asthma and rhinitis
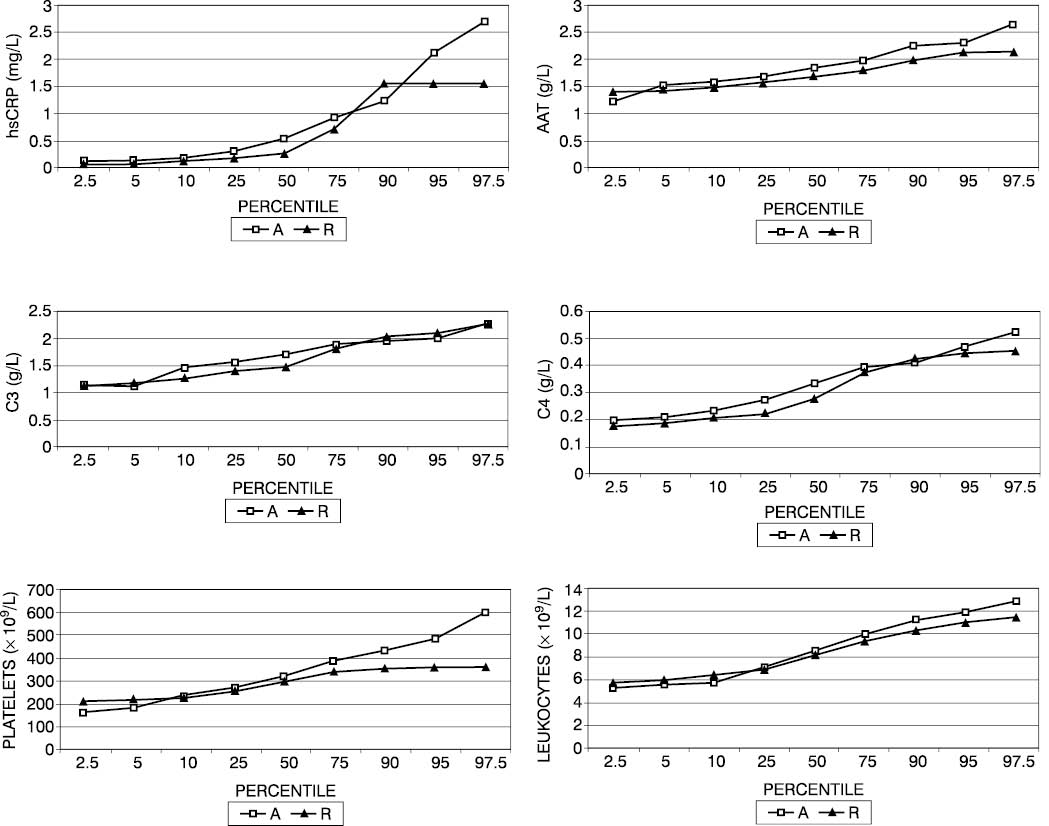
Percentile values revealed the patients with allergic rhinitis to have an hsCRP concentration of ≤1.57 mg/L, whereas 5% of asthma patients had an hsCRP concentration greater than 1.57 mg/L (Fig. 1). In some 40% of asthma patients, the concentration of the complement components C3 and C4 exceeded the concentration recorded in patients with allergic rhinitis. Platelet count was found to be ≤363x109/L in patients with allergic rhinitis and >450x109/L in 5% of asthma patients. Percentile values of AAT were by 8% on an average greater in asthma children than in those with allergic rhinitis.
Figure 1. Percentile values of hsCRP (a), AAT (b), C3 (c), C4 (d) concentrations, platelet count (e) and leukocyte count (f) in patients with asthma (A) and allergic rhinitis (R).
Discussion
The present study indicated the children with asthma and allergic rhinitis to have a higher concentration of hsCRP than healthy children. The search of the available literature revealed only one group of Israeli authors to have presented results of hsCRP determination in 63 asthma children. These authors compared hsCRP concentration in acute exacerbation of asthma and upon therapy administration, and found it to be significantly higher in acute disease as compared with post-therapeutic state (14.28±8.45 mg/L vs 1.92± 3.16 mg/L). They also report on the correlation between hsCRP concentration and forced expiratory volume in 1 second (FEV1) (18). In our study, both the children with asthma and those with allergic rhinitis had the mean hsCRP concentration lower than the concentration from the above mentioned report (0.71±0.58 mg/L and 0.53±0.50 mg/L, respectively). Takemura et al. (13) determined hsCRP concentration in adult asthmatic patients and showed it to be higher in patients without therapy with inhalation corticosteroids (1.33±1.48 mg/L) than either in healthy subjects (0.21±0.30 mg/L) or in patients receiving therapy (0.9±1.0 mg/L). The hsCRP levels recorded in our control group of healthy children (0.28±0.16 mg/L) were comparable to those reported by Takemura et al. (13) in healthy adults. According to some authors (19), systemic inflammation could also be verified in asthma patients, since these patients had an elevated concentration of acute phase proteins. Our study demonstrated the children with asthma and allergic rhinitis to have a higher leukocyte count and A1-AT concentration than healthy children, supporting the existence of mild systemic inflammation in patients with respiratory allergic diseases. In adult patients, it is not asthma alone that is the key factor to increase the concentration of hsCRP, as it can also be influenced by other factors such as the risk of cardiovascular disease (11), diabetes mellitus (12), obesity (20), atherosclerosis and atherothrombosis (21). The prevalence of these risk factors is by far lower in children; therefore, the elevated concentration of CRP in our children could have been ascribed to inflammation due to respiratory allergic diseases. It was demonstrated that complement also plays a role in allergic inflammation, as the C3 and C4 levels were greater in children with respiratory allergic diseases than in healthy controls. CRP is known to be able to activate complement components (22). Platelet count was also increased in patients with asthma but not in those with allergic rhinitis. Future studies should therefore investigate the causes of this difference between asthma and rhinitis because platelets may have a varying role in allergic reactions (23).
We are aware of the limitations of the present study due to the lack of information on the lipid status that may influence the hsCRP concentration. Study results (one of the first in this area) demonstrated that children with respiratory allergic diseases had greater concentrations of hsCRP in serum as compared with healthy children. Further studies are needed to demonstrate whether determination of hsCRP concentration could be useful in therapeutic monitoring of children with respiratory allergic diseases.
References
1. Kolbas V, Lokar R, Stanić M, Krznarić-Sučić Z. Prevalencija astme u djece školske dobi na području grada Zagreba. Arhiv Zast Majke Djeteta 1979;23:351-63.
2. Aberle N, Reiner-Banovac Z. Epidemiološko ispitivanje astme u djece. Pediatr Croat 1998;42:9-14.
3. Togias A. Rhinitis and asthma: evidence for respiratory system integration. J Allergy Clin Immunol 2003;111:1171-83.
4. Casale TB, Dykewicz MS, Clinical implications of the allergic rhinitis-asthma link. Am J Med Sci 2004;327:127-38.
5. Asthma management and prevention: a practical guide – 1996. (An information booklet for public health officials and health care professionals). NIH Publication No. 96-3659 B.
6. Global strategy for asthma management and prevention, 2002. Scientific information and recommendations for asthma programs. NIH Publication No. 02-3659.
7. Čepelak I, Dodig S, Štraus B, Labar B. Medicinsko-biokemijske smjernice. Zagreb: Medicinska naklada, 2004; str. 89.
8. Pepys MB, Baltz MC. Acute phase proteins with special reference to C-reactive protein and related proteins (pentaxins) and serum amyloid A protein. Adv Immunol 1983;34:141-212.
9. Silverman LM, Christenson RN. Amino acid and proteins. U: Burtis CA, Ashwood ER, ur. Tietz Fundamentals of clinical chemistry, 4. izdanje. Philadelphia: WB Saunders Company, 1996; str. 240-82.
10. Whicher J. C-reactive protein (CRP). U: Thomas L, ur. Clinical laboratory diagnostics. Prvo izdanje. Frankfurt/Main: TH-books, 1998; str.700-6.
11. Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation 2003;107:363-9.
12. Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001;286:327-34.
13. Takemura M, Matsumoto H, Niimi A, Ueda T, Matsuoka H, Yamaguchi M, Jinnai M, Mauro S, Hirai T, Ito Y, Nakamuro T, Mio T, Chin K, Mishima M. High sensitivity C-reactive protein in asthma. Eur Respir J 2006;27:908-12.
14. World Medical Association Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects, August 2005. Available at http://www.wma.net/e/policy/b3.htm).
15. Dupuy AM, Badiou S, Descomps B, Cristol JP. Immunoturbidimetric determination of C-reactive protin (CRP) and high sensitive CRP on heparin plasma. Comparison with serum determination. Clin Chem Lab Med 2003;41:948-9.
16. Zar JH. Biostatistical analysis, 2. izd. Englewood Clifts, NJ: Prentice-Hall, 1984.
17. MedCalc Download, available June 15, 2006, www.medcalc.be/download.php.
18. Soferman R, Gladshtein M, Weisman Y. C-reactive protein levels, a measurement of airway inflammation in asthmatic children. XXV Congress of the European Academy of Allergology and Clinical Immunology, Vienna, Austria, June 10-14, 2006. Abstract Book, str. 59.
19. Jousilahti P, Salomaa V, Hakala K, Rasi V, Vahtera E, Palosuo T. The association of sensitive systemic inflammation markers with bronchial asthma. Ann Allergy Asthma Immunol 2002;89:381-5.
20. Visser M, Bouter LM, McQuillen GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA 1999;282;2131-5.
21. Pepys MB. CRP or not CRP? That is the question. Arterioscler Thromb Vasc Biol 2005;25:1091-4.
22. Wolbink GJ, Brouwer MC, Buysmann S, tenBerge IJ, Hack CE. CRP-mediated activation of complement in vivo: assessment by measuring circulating complement-C-reactive protein complexes. J Immunol 1996;157:473-9.
23. Sullivan PJ, Jafar ZH, Harbinson PL, Restrick LJ, Costello JF, Page CP. Platelet dynamics following allergen challenge in allergic asthmatics. Respiration 2000;67:514-7.