Introduction
Preeclampsia is a complex multisystem disorder that is associated with hypertension, edema and proteinuria during pregnancy. An adaptive mechanism enhancing the maternal antioxidant defense system to counteract the effect of free radicals through enzymatic induction as well as through nonenzymatic free radical protectors and scavengers like reduced glutathione can prevent the occurrence of oxidative stress. However, pregnancy is a state where this adaptation may be easily disrupted.
Consensus does not exist whether the activities of the antioxidant enzymes superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase increase or decrease in preeclampsia. Several authors have reported a decrease in the activity of SOD, GPx and GRx (1-4), whereas others found their activities to increase (5-7). Similarly, the activity of catalase has been reported to increase (1,8), or suggested to decrease in preeclampsia (9).
Our laboratory has for long been involved in pursuing studies on pathological blood. Considering the contradictory reports and the seriousness of preeclampsia, the present study was carried out to determine the activities of catalase, SOD, GPx and glutathione reductase (GRx) in the blood of normal and severely preeclamptic women, along with the levels of malondialdehyde (MDA) and oxidized glutathione/reduced glutathione (GSSG/GSH) to collectively evaluate the alteration in pro-oxidant and antioxidant balance.
Literature reveals very little information on preeclamptic erythrocyte osmotic fragility, and this parameter is also important to assess the pathophysiological status of preeclampsia. There is a report on decreased GSH content in red blood cells of preeclamptic women, which has been correlated to the increased osmotic fragility and consequently reduced cellular deformability and membrane fluidity (10). Thus, we also included this parameter in our study, which yielded very interesting results.
Subjects and methods
Subjects
In India, pregnant women are encouraged to attend regular antenatal check ups. Standard antenatal care is defined as monthly visits up to 28 weeks, fortnightly until 34 weeks, and weekly visits thereafter. The present study was carried out with prior approval from the local Ethics Committee. The study included pregnant women with normal blood pressure as a control group and preeclamptic women admitted to our hospital that had been or had not been under regular care and also those referred from private sectors or primary health centers. Twenty-five normal pregnant women and 27 severely preeclamptic patients with term pregnancy were selected. They gave their consent in writing and the objectives of the study were fully explained to them in detail prior to taking consent. Body height and weight of the subjects were measured to calculate their body mass index (BMI). Clinical examination and history taking excluded women addicted to tobacco, patients with diabetes, ischemic heart disease, a history of stroke, kidney diseases or other conditions of known free radical etiology. The criteria for dividing women into normal primipara and preeclamptic primipara groups were set at a blood pressure of 140/90 mm Hg or higher, proteinuria and edema.
Methods
ATP, NADPH, GSH, GSSG, glutathione reductase, EDTA, TBA and butylated hydroxytoluene (BHT) were obtained from Sigma Chemical Company (St. Louis, MO, USA). Other chemicals were from E. Merck (Mumbai, India). All other reagents were of analytical grade, either from BDH or SISCO Chemicals (Mumbai, India).
Determination of osmotic fragility (OF)
Osmotic fragility (OF) experiments were performed following the method of Dacie and Lewis (11).The NaCl concentration of 50% hemolysis was taken as a measure of mean erythrocyte fragility (MEF). Color measurement was made using Systronics colorimeter.
Blood samples were centrifuged at 1000xg for 15 min at 4 °C and isolated red cells were washed 4–5 times with 0.154 M NaCl to remove plasma and buffy coat. After final wash, the required packed red cells were lysed by hypotonic shock and different dilutions were used as hemolysates.
Hemoglobin content of the erythrocyte was measured by cyanmethemoglobin method of Drabkin (12).
Determination of reduced glutathione (GSH)
Packed red cells (0.2 mL) were used in the assay. GSH was made to react with 5’5-dithiobis(2-nitrobenzoic acid), which reacts with sulfhydryl groups, to develop a stable color. The absorbance was measured at 412 nm and GSH content expressed as μmol/gHb (13).
Determination of oxidized glutathione (GSSG)
Erythrocyte lysate was deproteinized with 0.5M HClO4. Then estimation was made on the basis of reduction of GSSG in the presence of NADPH and glutathione reductase (GRx), and decrease of NADPH at 340 nm after initiating the reaction by adding GRx was taken as an index of GSSG content, which was evaluated and expressed as μmol/gHb (14).
Determination of lipid peroxidation
Packed red cells (0.2 mL) were used for determination of malondialdehyde (MDA) as thiobarbituric acid reactive substances (TBARS) employing the method of Jain et al. (15).
Uric acid determination
Simple colorimetric method of Buchanan et al. (16) was employed.
Determination of GPx (EC 1.11.1.9) activity
GPx activity was measured spectrophotometrically (17) at 340 nm in 50 mM phosphate, 5 mM EDTA, pH 7.0 containing 0.3 mM NADPH, 0.3 U/mL GRx, 5 mM GSH, 4 mM sodium azide, 75 μM H2O2 and 10 μL of erythrocyte lysate in a final reaction mixture of 3 mL. The hemolysate was pretreated with Drabkin’s reagent to produce stable cyanmethemoglobin, eliminating methemoglobin-reductase-mediated (or nonenzymatic) oxidation of NADPH. One unit of GPx was considered to be the amount necessary to oxidize 1 μmol NADPH/min. The activity was expressed as U/gHb.
Determination of SOD (EC 1.15.1.1) activity
SOD activity was measured according to the method of Beutler (13). Briefly, the reaction is dependent on the presence of superoxide anions that cause the oxidation of pyrogallol. The inhibition of pyrogallol oxidation by SOD was monitored and the amount of enzyme producing 50% inhibition was defined as one unit of enzyme activity. The assay mixture contained 1 M Tris, 5 mM EDTA buffer, pH 8.0, and 10 mM pyrogallol. The inhibition of pyrogallol oxidation by SOD was monitored at 420 nm, and the enzyme activity was evaluated and expressed as U/gHb.
Determination of catalase (EC 1.11.1.6) activity
Catalase decomposes H2O2 and forms water and molecular oxygen. H2O2 absorbs maximum light at 240 nm. The absorbance decreases as H2O2 is being decomposed by catalase. Determination of catalase activity was assayed by monitoring the rate of H2O2 decomposition spectrophotometrically at 240 nm following the procedure of Aebi (18). The assay mixture contained 0.9 mL of 1M Tris, 5 mM EDTA buffer, pH 7.0 and 0.1 mL of the sample. The reaction was started by adding 1.0 mL of 200 mM hydrogen peroxide (H2O2) in the test cuvette and by adding the same volume of distilled water instead of hydrogen peroxide in the reference cuvette. The decrease in absorbance was measured with a recorder at an interval of 30 seconds for 3 minutes. The value of absorbance of the reference cuvette was subtracted from that of the test cuvette before calculating the units of activity. The activity of catalase was evaluated and expressed as kU/gHb.
Determination of GRx (EC 1.8.1.7) activity
The main reagent was prepared by combining 18 mL of KH2PO4 buffer 139 mM, 0.76 mM EDTA, pH 7.4 and 2 mL of NADPH 2.5 mM. The sample (20 μL of 1: 20 hemolysate + 20 μL of KH2PO4 buffer), 220 μL of the main reagent and 5 μL of FAD 0.315 mM + 10 μL of KH2PO4 buffer were added to the cuvette and the absorbance was monitored at 340 nm for 200 s (step A). Then 30 μL of GSSG 22 mM + 10 μL of KH2PO4 buffer were added to start the reaction and the absorbance was followed for 175 s (step B). The final reaction volume was 315 μL. The difference in absorbance per minute between steps B and A was used to calculate the enzyme activity. The unit was μmol of NADPH oxidized/min and the GRx activity was evaluated and expressed as U/gHb (19).
Statistical analysis
Data were expressed as mean ± standard deviation. Student’s t-test was performed for statistical analysis of data to compare normotensive control and preeclamptic patient groups. The two-tailed probability P-values were calculated using GraphPad QuickCalcs Software. The t-test statistical significance was set at P ≤ 0.05.
Results
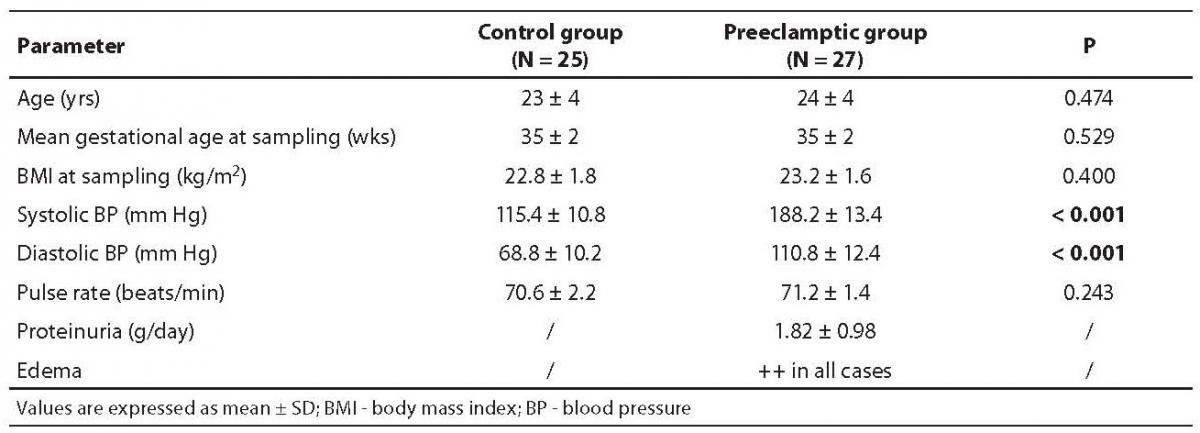
Demographic and clinical parameters of the normotensive healthy subjects and patients with severe preeclampsia are summarized in Table 1. It is evident from clinical findings that the average blood pressure was very high in preeclamptic women, indicating the severity of preeclampsia.
Table 1. Demographic and clinical parameters of normotensive (control group) and severe preeclampsia subjects
Alterations in pro-oxidant and antioxidant nonenzymatic metabolites and osmotic fragility profiles
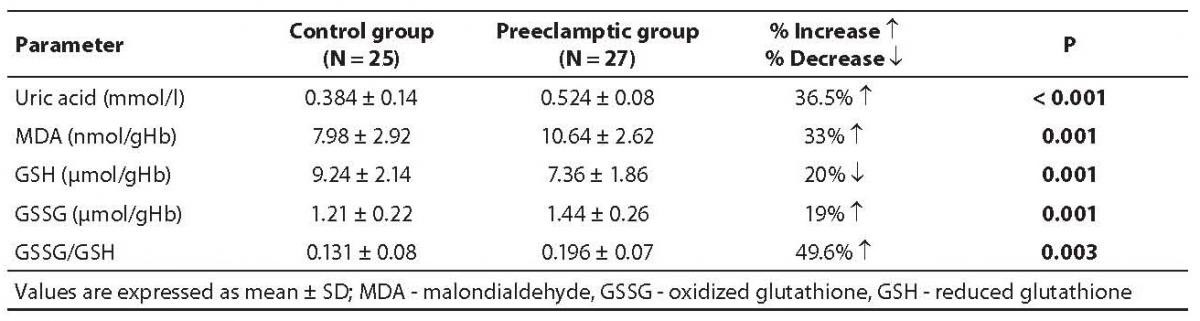
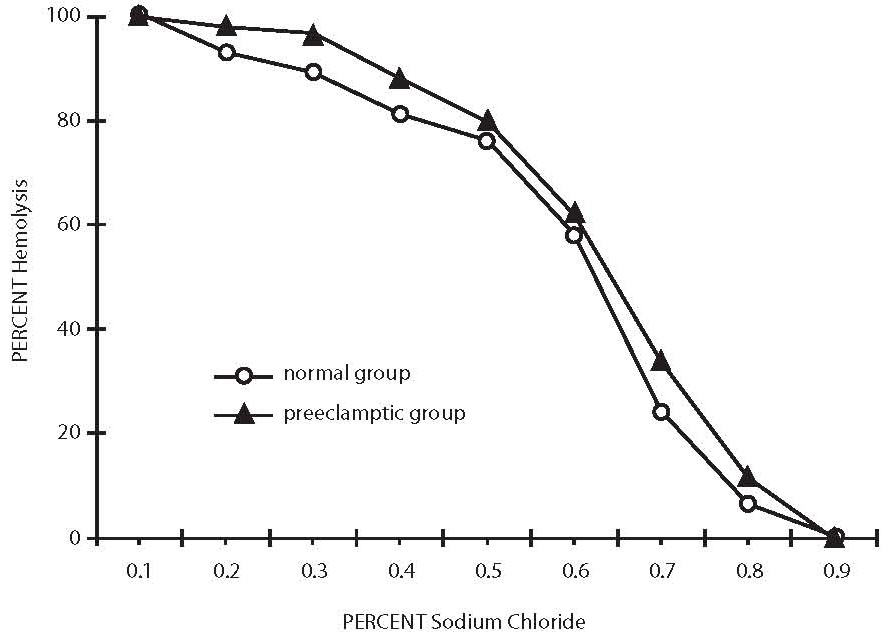
All oxidant parameters were significantly higher in preeclamptic women when compared with controls, with the exception of GSH, which was significantly decreased, pointing to the enhanced oxidative stress in severe preeclampsia (Table 2). The erythrocytes from preeclamptic patients underwent higher lysis than those from normal primiparae. In patients with severe preeclampsia, the osmotic fragility profile showed a shift to the right from the normotensive one due to their increased erythrocyte osmotic fragility (P= 0.001). The osmotic fragility profiles are depicted in Figure 1.
Table 2. Oxidant and antioxidant contents in control and severe preeclampsia subjects
Figure 1. Osmotic fragility profiles of normal healthy control and severe preeclampsia patients.
Alterations in the enzymatic antioxidant status
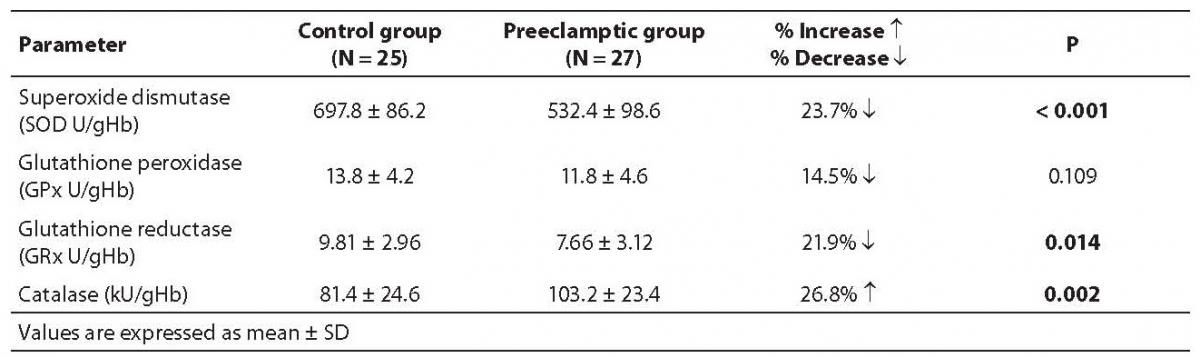
We evaluated the quantum of enzymatic antioxidant defense both in normal and severely preeclamptic women, and found much variation in their profiles (Table 3). The antioxidant enzymes (SOD, GRx and GPx) showed a decrease in their activities in preeclamptic women as compared with normal women, indicating the loss in their antioxidant capacity, whereas a significant increase in catalase activity showed its compensating regulatory role in response to the increased oxidative stress.
Table 3. Activities of various antioxidant enzymes in control and severe preeclampsia subjects
Discussion
In the present study, we evaluated the alterations in pro-oxidant and antioxidant balance in severe preeclampsia by determining the levels of nonenzymatic scavengers like reduced glutathione, antioxidant enzymatic activities and major metabolites of lipid peroxidation. We also evaluated erythrocyte osmotic fragility that is directly related to the pathophysiological conditions of preeclampsia. We did so because red blood cells are particularly susceptible to oxidative damage as they act as an oxygen carrier (getting exposed to high oxygen tension); do not have the capacity to repair themselves; their membranes are prone to lipid peroxidation; and hemoglobin is more susceptible to auto-oxidation.
Our present investigation revealed a significant increase in erythrocyte MDA concentration in severely preeclamptic patients in comparison to normal controls. This may result in a greater potential for endothelial damage ultimately leading to elevated diastolic pressure (20), which further aggravates the condition of preeclamptic patients (4). Enhanced ROS in turn can oxidize many other important biomolecules including erythrocyte membrane phospholipids. As mentioned before, there are literature reports on increased levels of MDA or TBARS in preeclampsia. Thus, our findings are consistent with those reported elsewhere (4,20).
The role of reduced glutathione in the protection of macromolecules against oxidative damage was clearly evident from our findings since the level of GSH was significantly higher in normotensive women as compared with preeclamptic women. Reduced glutathione provides resistance to cells against oxidative insult with sufficient intracellular concentration of GSH. During oxidative insult, GSH is oxidized to GSSG, as a consequence of which the level of GSSG increases. However, total level of GSH and GSSG was decreased significantly in preeclamptics, which may be due to defective synthesis of GSH in erythropoiesis or increased export of GSSG from preeclamptic erythrocytes. The enhanced efflux of GSSG seems to be one of the reasons, as according to Srivastava and Beutler (21) human erythrocytes transport GSSG at high levels of intracellular GSSG and red blood cell membranes transport GSSG but not GSH.
The erythrocyte GSSG/GSH ratio may serve as an early and sensitive parameter of the oxidative imbalance and a relevant target for future clinical trials to control the effects of antioxidant treatment in women at an increased risk of the preeclampsia syndrome (22). Our data clearly indicated that red blood cell GSH decreased profoundly in the pathophysiological condition of preeclampsia with a parallel increase in MDA and GSSG concentration which is in agreement with those reported in preeclampsia by Padmini and Geetha (23) and Yoshio et al. (24).
We observed that increased lysis resulted from oxidative damage to the erythrocyte membrane, causing a decrease in membrane fluidity and reducing its ability to withstand osmotic changes, and intracellular glutathione was more oxidized in erythrocytes from preeclamptic women as compared to normotensive primiparae. Our observation is in harmony with the report on preeclampsia by Spickett et al. (10). We have previously reported increased osmotic fragility of diabetic erythrocytes, which yielded normal osmotic fragility profile on insulin treatment (25). The main determination of in vitro hemolysis is the volume of the cell at any given time in relation to its maximal possible membrane surface area. In vitro osmotic fragility is dependent on: i) the suspending medium, whose pH and tonicity are controlled in the osmotic fragility test; ii) total number of intracellular osmotically active constituents, which determine cell volume in any given external environment; and iii) the critical hemolytic volume, a complex parameter dependent on quantitative and qualitative factors associated with the membrane lipid and protein. Therefore, the important relationship determining osmotic fragility is the ratio of critical hemolytic volume to the internal osmotic contents of the red blood cell. Our results on the red cell contents of GSH, GSSG and MDA in normal and preeclamptic women clearly pointed to a significant change in their internal contents. We have reported (26) that, when the erythrocyte loses the ability to maintain its GSH concentrations, the membrane proteolytic mechanism becomes active, causing sialoglycopeptide release and surface modifications that enable hemocatheretic organs to remove old cells from the blood circulation.
In the present study, the erythrocyte SOD and GRx antioxidant enzyme activities decreased significantly. SOD is an important antioxidant enzyme having an antitoxic effect against superoxide anion and catalyzing the reaction in which superoxide radicals are converted to H2O2 and O2. It decreases superoxide anion concentration in the vascular cell (27), a mechanism that could counteract the development of hypertension. Our results showed the failure of both these enzymes to perform their roles in case of preeclampsia up to the required extent because of their reduced activities. Glutathione peroxidase (GPx), an oxidative stress inducible enzyme, plays a significant role in the peroxyl scavenging mechanism and in maintaining the cell membrane integrity (28). Its activity decreased in preeclampsia, although non-significantly, which could be interpreted as not providing protection to the red cell membrane integrity. Our findings on the activities of SOD, GPx and GRx in preeclampsia are in harmony with the reports of others (1-4). Contrast reports of an increase in their activities (5-7) might be explained on the basis of the lack of vitamin E levels in the study subjects, which depends on the severity of preeclampsia. During normal pregnancy, plasma vitamin E concentrations show progressive elevation, what could be due to the gestational increase in circulating lipoproteins as vitamin E transporters. In patients with mild preeclampsia, maternal blood α-tocopherol concentrations were not decreased as compared with normal pregnancies (29,30), but in patients with severe preeclampsia plasma α-tocopherol was significantly decreased as compared with controls, which is thought to be caused by the fact that antioxidants may be utilized to a greater extent to counteract free radical-mediated cell disturbances, resulting in a reduction in their plasma levels (31). The significant elevation in preeclamptic catalase activity shows the protective effect of this enzyme, which protects the cells from the accumulation of H2O2 by dismutating it to form water and oxygen by using it as an oxidant in which it works as a peroxidase (32).
In conclusion, we hypothesize the oxidative stress to be increased in preeclampsia, based on our results showing decreased SOD, GRx and GPx activities, which failed to control higher oxygen free radical production therein. The increased activity of catalase may be a compensatory regulation in response to the increased oxidative stress. The increased catalase activity could be interpreted as a futile effort to counteract the overproduction of reactive oxygen species and providing relief to the increased oxidative damage in preeclampsia. However, the increased osmotic fragility clearly indicated the loss in cellular membrane integrity and shortened life span of preeclamptic red blood cells. Lipid peroxides could be a part of the cytotoxic mechanism leading to the endothelial injury and elevated blood pressure. Finally, our findings suggested pro-oxidants to prevail over antioxidants in preeclampsia and the balance was ultimately disturbed in favor of oxidative stress, which was not the causative factor but the consequence of preeclampsia development. Further studies on the effect of antioxidant therapy, to combat the oxidative burden, may be more helpful to understand properly the mechanism of the development of pathophysiological conditions of preeclampsia.
Acknowledgment
We are highly grateful to those patients of our hospital who volunteered to donate their blood when needed for this project. The authors would also like to thank the paramedical staff of this hospital for their assistance in collection and storage of blood samples.
Notes
Potential conflict of interest
None declared
References
1. AtamerY, KocyigitY, YokusB, AtamerA, ErdenAC. Lipid peroxidation, antioxidant defense, status of trace metals and leptin levels in preeclampsia. Eur J Obstet Gynecol Biol 2005;119:60-6.
3. Yildirim A, Altinkaynak K, Aksoy H, Sahin YN, Akcay F. Plasma xanthine oxidase, superoxide dismutase and glutathione peroxidase activities and uric acid levels in severe and mild preeclampsia. Cell Biochem Funct 2004;22:213-7.
4. Chamy VM, Lepe J, Catalan A, Retamal D, Escobar JA, Madrid EM. Oxidative stress is closely related to clinical severity of preeclampsia. Biol Res 2006;39:229-36.
5. Llurba E, Gratacos E, Martin-Gallan P, Cabero L, Dominguez C. A comprehensive study of oxidative stress and antioxidant status in preeclampsia and normal pregnancy. Free Radic Biol Med 2004;37:557-70.
6. Orhan H, Onderoglu L, Yiicel A, Sahin G. Circulating biomarkers of oxidative stress in complicated pregnancies. Arch Gynecol Obstet 2003;267:189-95.
7. ark MC. The maternal change of malondialdehyde levels in plasma and superoxide dismutase levels in plasma and erythrocyte as biologic markers of oxidative stress in pregnancy with preeclampsia. Korean J Obstet Gynecol 2005;48:2550-7.
8. Noyan T, Guler A, Sekeroglu MR, Kamaci M. Serum advanced oxidation protein products, myeloperoxidase and ascorbic acid in preeclampsia and eclampsia. Aust N Z J Obstet Gynaecol 2006;46:486-91.
9. Kumar CA, Das UN. Lipid peroxides, anti-oxidants and nitric oxide in patients with pre-eclampsia and essential hypertension. Med Sci Monit 2000;6:901-7.
10. Spickett CM, Reglinski J, Smith WE, Wilson R, Walker JJ, Mckillop J. Erythrocyte glutathione balance and membrane stability during preeclampsia. Free Radic Biol Med 1998;24:1049-55.
11. Dacie JV, Lewis SM. Practical hematology. New York: Churchill-Livingstone, Inc., 1984; pp. 152-6.
12. Tentori L, Salvati AM. Hemoglobinometry in human blood. Methods Enzymol 1981;76:707-15.
13. Beutler E. Red cell metabolism. A manual of biochemical methods. 3rd ed. New York: Grune and Stratton, Inc., 1984.
14. Videla LA, Junqueira VBC. Metabolism of hepatic glutathione. Laboratory exercise I. 1-6. In: Oxygen radicals in biochemistry, biophysics and medicine. International Training Course. School of Pharmacy and Biochemistry, Buenos Aires, University of Buenos Aires, Argentina, 1994; pp. 7-18.3.
15. Jain SK, McVie R, Duett J, Herbst JJ. Erythrocyte membrane lipid peroxidation and glycosylated hemoglobin in diabetes. Diabetes 1989;38:1539-43.
16. Buchanan MJ, Isdale IC, Rose BS. Serum uric acid estimation. Chemical and enzymatic methods compared. Ann Rheum Dis 1965;25:285-8.
17. Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 1967;70:158-69.
18. Aebi H. Catalase in vitro. Methods Enzymol 1984;105:121-6.
19. Goldberg DM, Spooner RJ. Glutathione reductase. In: Bergmeyer HU, ed. Methods Enzymol Basel Verlag Chemie 1983;3:258-65.
20. Aydin A, Benian A, Modazli R, Ulodag S, Uzun H, Kaya S. Plasma malondialdehyde, superoxide dismutase, sE-selectin, fibronectin, endothelin-1 and nitric oxide levels in women with preeclampsia. Eur J Obstet Gynecol Reprod Biol 2004;113:21-5.
21. Srivastava SK, Beutler E. The transport of oxidized glutathione from human erythrocytes. J Biol Chem 1969;244:9-16.
22. Nemeth I, Orvos H, Boda D. Blood glutathione redox status in gestational hypertension. Free Radic Biol Med 2001;30:715-21.
23. Padmini E, Geetha BV. Placental heat shock protein 70 overexpression confers resistance against oxidative stress in preeclampsia. Turk J Med Sci 2008;38:27-34.
24. Yoshio Y, Rintaro S, Shunji S. Daisuke D, Koichi Y, Yasuo O, et al. Relationship between plasma malondialdehyde levels and adenosine deaminase activities in preeclampsia. Clin Chim Acta 2002;322:169-73.
25. Suhail M, Rizvi SI. Red cell membrane (Na++K+)-ATPase in diabetes mellitus. Biochem Biophys Res Commun 1987;46:179-86.
26. Brovelli A, Suhail M, Pallavicini G, Sinigaglia F, Balduini C. Self-digestion of human erythrocyte membranes. Role of adenosine triphosphate and glutathione. Biochem J 1977;64:469-72.
27. Chen X, Touyz RM, Park JB, Schiffrin EL. Antioxidant effects of vitamin C and E are associated with altered activation of vascular NADPH oxidase and superoxide dismutase in stroke-prone SHR. Hypertension 2001;38:606-11.
28. Chandra R, Aneja R, Rewal C, Konduri R, Dass SK, Agarwal S. An opium alkaloid-papaverine ameliorates ethanol induced hepatotoxicity: diminution of oxidative stress. Indian J Clin Biochem 2000;15:155-60.
29. Mikhail MS, Anyaegbunam A, Garfinkel D, Palan PR, Basu J, Romney SL. Preeclampsia and antioxidant nutrients: decreased plasma levels of reduced ascorbic acid, alpha-tocopherol and beta-carotene in women with preeclampsia. Am J Obstet Gynecol 1994;17:150-7.
30. Sagol S, Ozkinay E, Ozsener S. Impaired antioxidant activity in women with preeclampsia. Int J Gynaecol Obstet 1999;64:121-7.
31. Kharb S. Vitamin E and C in preeclampsia. Eur J Obstet Reprod Biol 2000;93:37-9.
32. Lenzi A, Culasso F, Gandini L, Lombardo F, Dondero F. Andrology: placebo controlled, double-blind, cross over trial of glutathione therapy in male infertility. Hum Reprod 1993;8:1657-62.