Introduction
Antioxidant defense mechanisms of the body include cellular and extracellular enzymes and free radical quenchers (like vitamins C, E, and A). Cord blood antioxidant capacity is the result of overall intrauterine experience. Genetic variability, maternal oxidative stress, and maternal antioxidant capacity are likely to alter the cord antioxidant capacity. After birth, increased oxidative stress will result in a decreased antioxidant capacity. Once the antioxidant capacity is overwhelmed, then an increase in oxidation products of lipids, protein, and nucleic acids is expected, resulting in tissue damage. Clinical manifestations of oxygen radical diseases depend on a balance between tissue damage and repair. Our aim was to assess the relationship between lipid peroxidation and maternal/cord blood antioxidant capacity and to examine whether the cord blood with oxygen radical disease had different total antioxidant capacity than the one without preeclampsia, a disorder of pregnancy characterized by pregnancy-induced hypertension (≥140 mmHg systolic and / or ≥ 90 mmHg diastolic blood pressure), new-onset proteinuria (≥ 300mg protein/day), and edema occurring in the second half of pregnancy.
Significant elevation of malondialdehyde (MDA) levels in the cord of pair-matched preeclamptic mother has been reported (1,2), whereas some authors observed a decline in its level (3) or no significant change (4). Karabulut et al. (5), however,inferred elevation of MDA levels both in the cord and mother during preeclamptic development compared to normal pregnancy. During normal pregnancy, several reports showed lower cord vitamins A, E contents (6,7) but Kanishtha et al. (8) reported higher vitamin Alevel in the cord plasma. There are reports (9,10) showing significantly higher vitamin E level in the cord plasma of the preeclamptic mother, whereas some authors observed (2)significantly lower cord serum vitamin E level as compared to maternal preeclamptics. Similarly, contradictions exist about the level of vitamin C in the cord blood of preeclamptic mothers. Some authors (12,13) reported higher contents of vitamin C in preeclamptic plasma in contrast to lower levels in the cord (11,2), whereas others (14) reported no change in the vitamin C level of a preeclamptic mother.
Inconsistency in these reports has led us to take up the present study in order to understand and elaborate if total antioxidant capacity increases or decreases during preeclampsia. Further, we agree with the advocacy of Chappell et al. (15) that there is a need to perform large multicenter trials to (a) measure the levels of oxidative stress markers and antioxidants, (b) administer antioxidant therapy to all women with abnormal levels, and (c) determine whether these levels can be improved with antioxidant therapy. We have recently reported our findings on these aspects along with the effects of vitamins E and C (16,17). The aim of the present study was to assess the relationship between lipoperoxidation and maternal/cord blood antioxidant capacity and to examine whether the maternal and cord plasma concentrations of malondialdehyde, vitamins E, A, and C differ between preeclamptic and healthy pregnant women.
Materials and methods
Subjects
The patients in our study included pregnant women (N = 21) with normal blood pressure, severely preeclamptic women (N = 21) admitted to our hospital, who either had or not been under regular care, and those who were referred from private sectors or primary health centers. The subjects of both groups were not administered any multivitamin supplementation. All the participants were within the age range of 18-34 years. The preeclamptic women had the blood pressure ≥ 160/110, with urinary protein excretion over 0.8 g/24 hours and edema. The present study was carried out with the prior approval of the local ethic committee. All the patients mentioned above gave their consent in writing, and the objectives of the study were fully explained to them in detail prior to signing consent. Clinical examination and history taking excluded pregnant women addicted to tobacco smokers, patients with diabetes, ischemic heart disease and a history of stroke, kidney disorders or other conditions of known free radical etiology.
Samples
Blood samples were collected from mothers at delivery. The cord blood was obtained immediately post partum from the umbilical vein after clamping of the cord by labor ward staff. In each case, 10 mL blood were drawn into a sodium heparin vacutainer tube for separating plasma and stored at 4 ºC until processed. Maternal and umbilical cord blood samples were handled identically, all samples were processed within 20 hours of sampling, and plasma samples were stored at -70 ºC until required for vitamin analyses. Before storage, an equal volume of metaphosphoric acid (10%) was added to plasma samples designated for vitamin C analysis in order to deproteinize the plasma and stabilize vitamin C content.
All the chemicals were of analytical grade from either E. Merck (Mumbai, India), BDH or SISCO Chemicals (Mumbai, India).
Methods
Estimation of lipid peroxidation
Lipid peroxidation was quantified was carried out following the method of Jain et al. (18). Packed red cells (0.2 mL) were used for the quantification of malondialdehyde (MDA) as thiobarbituric acid reactive substances (TBARS). Aliquots of 0.2 mL were mixed thoroughly with 0.8 mL of phosphate buffered saline (pH 7.4) and 25 μL of butylated hydroxytoluene solution. After adding 0.5 mL of 30% trichloroacetic acid, the samples were placed on ice-bath for 2 hrs and then centrifuged at 2000 g at 25 ºC for 15 min. One mL of supernatant was mixed with 75 μL of 0.1 M EDTA and 250 μL of 1% thiobarbituric acid in 0.05 M NaOH and placed on boiling water for 15 min. After cooling to room temperature, absorbance was measured at 532 nm. MDA contents were expressed as nmol/gHb.
The linearity established for MDA concentrations ranged from 0.2-6 µmol/L, accuracy/recovery percentage was 90-95%, precision coefficient of variation (CV) values were 5% (intraday) and 12% (inter-days). LOD (limit of detection) and LOQ (limit of quantification) were 0.05 µmol/L and 0.09 µmol/L, respectively.
Estimation of plasma vitamin C
Vitamin C concentrations were determined in plasma using the method of Jagota and Dani (19). 0.2 mL of plasma was precipitated on ice with 0.8 mL of trichloroacetic acid for 5 minutes and then centrifuged at 1000 g for 5 min. A total of 0.5 mL of the supernatant was diluted with distilled water to the volume of 2 mL. 200 μL of Folin-Ciocalteau’s solution were diluted 1:10 in distilled water and added to the samples which were immediately mixed. After 10 min, the absorbance at 760 nm was measured spectrophotometrically. The sample values were compared with values of standard samples of ascorbic acid prepared in distilled water.
The linearity established for vitamin C concentrations ranged from 2-40 mg/mL, accuracy/recovery percentage was 93-100%, precision CV values were 2.5% (intraday) and 4.5% (inter-days). LOD and LOQ were 0.5 mg/L and 1 mg/L, respectively.
Estimation of plasma vitamin A
This estimation was performed following the procedure of Sobel and Snow (20). 1 mL of 95 per cent ethanol was added to 1 mL of plasma, and the contents of the tube mixed by tapping. 2 mL of analytical reagent petroleum ether was added, and the tube was shaken for 10 min. After shaking, the tube was centrifuged for about 30 seconds. The supernatant petroleum ether was aspirated and placed in a test tube. With another 2 mL of the petroleum ether, and shaking for only 5 min, the extraction procedure was repeated. The extract was evaporated to dryness by placing the tube in a 40-50 oC water bath and running a stream of nitrogen over it. 1 mL of analytical reagent grade chloroform was added to bring the dried extract into solution. 4 mL of glycerol dichlorohydrin (GDH) was added. The chloroform solution and the GDH were mixed and after 2 min, absorption was measured first at 550 nm against a blank consisting of 4 mL of GDH and 1 mL of chloroform.
The linearity established for vitamin A concentrations ranged from 50–1200 µg/L, accuracy/recovery percentage was 87-96%, precision CV values were 7.5% (intraday) and 10.8% (inter-days). LOD and LOQ were 9 µg/L and 20 µg/L, respectively.
Estimation of plasma vitamin E
A volume of 0.8 mL of plasma was pipetted into a test tube and an equal volume of purified absolute ethanol was added to the tube for protein precipitation. The contents were immediately mixed with a vortex mixer. Then, 0.8 mL of xylene was added and the test tube was mixed for at least 30 sec and centrifuged for 5-10 min at 1000 g. After centrifugation, the upper xylene layer, which contained the extracted tocopherol, was collected with a medicinal dropper and transferred to a small tube. The tubes were covered with parafilm to avoid evaporation. Added 0.4 mL of plasma-xylene extract to the test tube containing 0.2 mL of 4, 7-diphenyl-1, 10-phenanthroline (bathophenanthroline, BA). A volume of 0.2 mL ferric chloride was added, followed by 0.2 mL of orthophosphoric acid. The contents of the tube were mixed thoroughly using a vortex mixer after every addition of reagents. The order of reagent addition is critical. Absorbance was read in the spectrophotometer at 536 nm after setting the instrument to zero absorbance with a blank (prepared by using 0.4 mL xylene instead of plasma-xylene extract).
The linearity established for vitamin E concentrations ranged from 0.8–20 mg/L, accuracy/recovery percentage was 85-90%, precision CV values were 6% (intraday) and 11% (inter-days). LOD and LOQ were 0.4 mg/L and 0.6 mg/L, respectively. Vitamin E contents were expressed as µmol/L (21).
Statistical analysis
SPSS version 15.0 for Windows (SPSS Inc., Chicago, IL, USA) software package was used to analyze the data of various parameters. The results were statistically analyzed using t-test to compare both maternal/cord blood of normotensive pregnant and preeclamptic patient groups. Sample pairs (maternal plasma and cord blood) in both normotensive and preeclamptic patient groups were compared using paired t-test. The statistical significance was set at P ≤ 0.05. Values were expressed as percentage and mean ± standard deviation.
Results
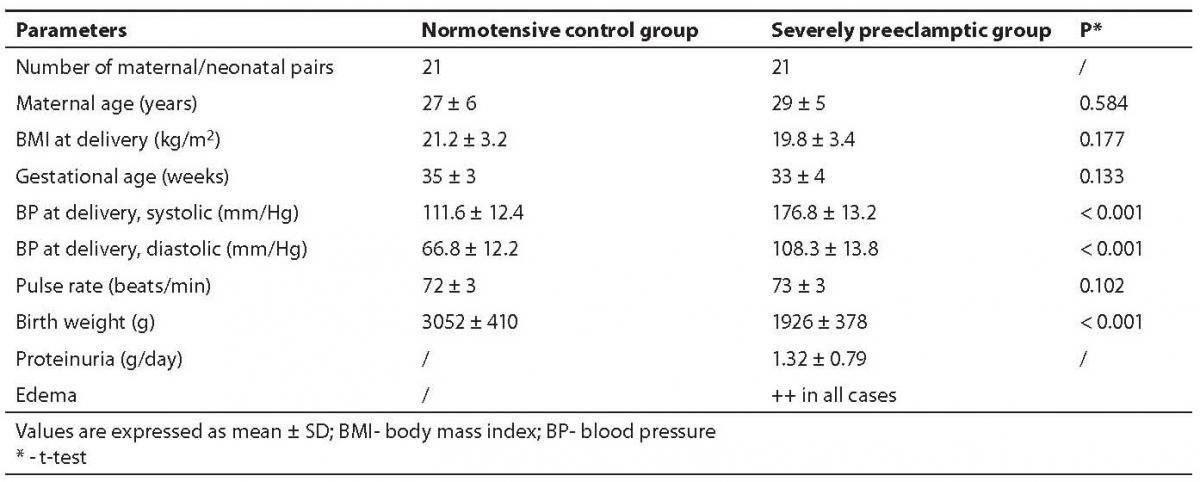
We compared the parameters of the oxidative and anti-oxidative system in maternal and cord blood of two groups of pair matched mothers and neonates. Group A consisted of 21 uncomplicated, normotensive- pregnancies (called controls) and group B comprised 21 severely preeclamptic patients, all singleton pregnancies. Group A had three, whereas group B had four caesarian sections because of prolonged labor. The characteristics of these groups are shown in Table 1.
Table 1. Characteristics of study groups
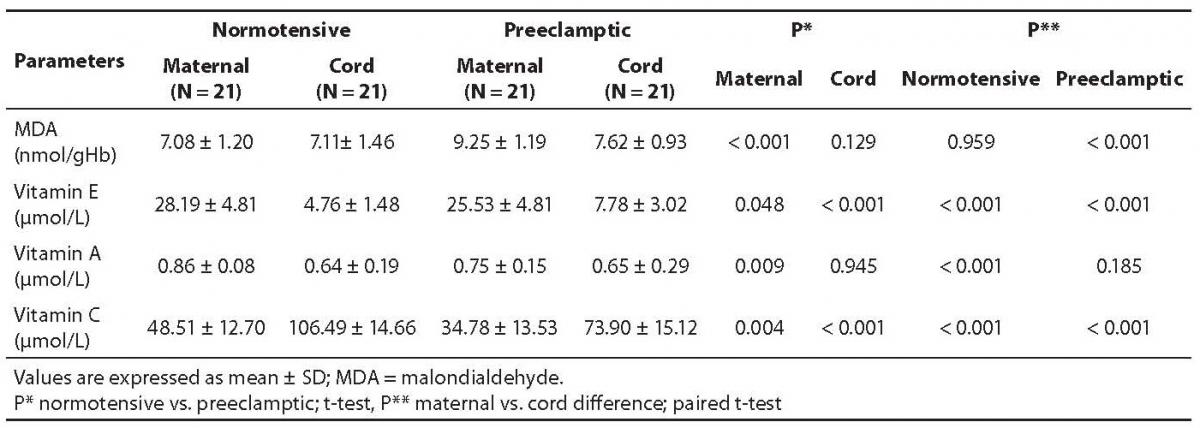
The mean values along with statistical significant differences of the normotensive and preeclamptic pair-matched maternal and cord plasma are shown in Table 2.
Table 2. Concentrations of malondialdehyde, vitamins E, A, and C in pair-matched normotensive and preeclamptic maternal and cord plasma.
The MDA content in preeclamptic maternal plasma was found to be 30.6% higher than that of control. There was no significant difference between control maternal and cord plasma levels. The MDA content in preeclamptic maternal plasma was significantly high (P < 0.001) compared to control. However, its content in preeclamptic cord plasma compared to maternal plasma was significantly low (P < 0.001) as evident from Table 2.
Vitamin E level was low in severely preeclamptic maternal plasma compared to normotensive mother which amounted to be by 9.4% lower than in control. The concentrations were significantly lower (P < 0.001) in both the cord plasma of controls, as well as those from preeclamptics.
Vitamin A amount was significantly low (P = 0.009) in preeclamptic maternal plasma compared to normotensive group. Interestingly, there was no significant difference between maternal and cord levels in severely preeclamptic patients. However, the cord plasma had significantly lower concentrations of vitamin A compared with those of maternal plasma. Table 2 depicts the variation in the mean values of both pair-matched groups.
Contrary to vitamins E and A, the concentrations of vitamin C in the cord plasma collected from both groups showed significantly higher (P < 0.001) levels compared to pair-matched maternal plasma. Its concentrations were significantly low (P = 0.004) in preeclamptic maternal plasma compared with those of normotensive subjects, but in preeclamptic cord plasma the level was significantly (P < 0.001) high. Its level was significantly (P < 0.001) higher in the normotensive cord compared to the preeclamptic cord.
Discussion
Cumulative evidence in recent years that a biochemical imbalance in preeclampsia occurs with an increase of oxidative stress and a deficient antioxidant protection (16,17,22). Antioxidant defense systems include the chain-breaking antioxidants, such as vitamin C and vitamin E, and antioxidant enzymes. Lipid-phase chain-breaking antioxidants, the most important of which is probably vitamin E (23), scavenge radicals in membranes and lipoprotein particles and are central to the prevention of lipid peroxidation. Aqueous-phase chain-breaking antioxidants directly scavenge radicals present in the aqueous compartment. Vitamin C or ascorbate is the most important aqueous phase chain-breaking antioxidant (24). It is well established that there is synergy between vitamins C and E. This interaction between vitamin C and vitamin E has been confirmed in vivo by authors (25) who have reported that supplementation of healthy adults with ascorbic acid increases ascorbic acid and lipid-standardized α-tocopherol levels in plasma, and that supplementation with α-tocopherol is associated with increased plasma ascorbic acid concentration, as well as improved vitamin E status.
Our findings showed significantly low vitamin E levels in the cord plasma compared to pair-matched preeclamptic maternal plasma, which is consistent to other reports (12). Similarly, our results on significantly low concentrations of vitamin A in the cord plasma of pair-matched normotensive mother are consistent with other reports (6, 7,11), but no significant change was observed in the case of preeclamptic maternal and cord plasma. Interesting results were observed with the vitamin C levels which were significantly higher in both the cords of normotensive as well as preeclamptic mothers, which is in harmony with other reports (6,12). However, the significantly low (P < 0.001) level in pair-matched preeclamptic mother can be explained because of high MDA concentration in preeclamptic mother, which might have consumed more of vitamin C, consequently resulting in the low amount of vitamin C.
MDA, a product of lipid peroxidation induced by ROS, is well correlated with the degree of lipid peroxidation (16,26). Our results show 30.6% higher MDA content in preeclamptics compared to normotensive maternal plasma. Earlier, it has been reported (27) that preeclamptic placenta contains higher MDA than those from normal pregnancies. However, 17.6% lower concentration of MDA in the cord plasma of preeclamptics compared to their pair-matched maternal plasma might be due to increased consumption of vitamin C. Present results on MDA content were consistent with our previous report (16) along with those by other authors (1,2,5).
Plasma antioxidant activities alter progressively throughout pregnancy, as mentioned earlier. Antioxidant vitamins, with the ability to stabilize highly reactive free radicals, act as the first line of defense against free radical attack and lipid peroxidation. Vitamins E (α-tocopherol) and C show differences in the contribution they make to antioxidant potential, as vitamin E is the major lipid soluble chain-breaking antioxidant in cell membranes while vitamin C is an important aqueous phase antioxidant. Antioxidants may act synergistically; for instance, when vitamin C regenerates α-tocopherol from the tocopherol radical (28) this ‘sacrificial’ antioxidant acts more by sparing vitamin E than by recycling it (24). The important role of vitamin C in preeclampsia suggests that changes in its concentration may influence susceptibility of vascular endothelium to oxygen toxicity (29). Thus, our present study on vitamin C concentration may provide a means of assessing the total capacity of the chain-breaking antioxidants to prevent lipid peroxidation in plasma and it might be important to evaluate the effectiveness of potential antioxidant defense systems on a limited scale.
In our previous report, we had concluded that antioxidant supplementation in women who were at risk of preeclampsia was associated with improvement in the activity of antioxidants, and use of vitamins C and E showed control of certain important biochemical indices during the development of preeclampsia (17). Further, present results are in harmony with our previous report on the role of vitamins C and E in severe preeclampsia. We infer that there is an imbalance between lipooxidation and antioxidant vitamins levels during pregnancy which is increased in severe preeclampsia, and overall oxidative status in the cord plasma of both pair-matched mothers are nearly the same. Thus, the preeclamptic mother is under oxidative stress rather than the pair-matched neonate.We hypothesize that the antioxidant capacity of the cord blood is sufficient, and placental barrier is adequate, to shield the fetus from oxidative injury due to increased oxidative stress of preeclamptic mother. Thus, we conclude that the oxidative stress status is low in the blood of neonates compared to its level in the pair-matched preeclamptic mothers. Further studies are needed to explore strategies to be applied so that the normal levels of antioxidant vitamins are maintained to combat preeclampsia in women at high risk.
Acknowledgment
We are highly grateful to those patients of our hospital who volunteered to donate their blood when needed for this project. The authors would like to thank Dr. Safia Suhail, Senior Gynecologist of our hospital for managing the patients. Our thanks are also due to the paramedical staff of this hospital for their assistance in collecting and maintaining blood samples.
Notes
Potential conflict of interest
None declared
References
1. Biri A, Buzkurt N, Turp A, Kavutcu M, Himmetoglu O, Durak I. Role of oxidative stress in intrauterine growth restriction. Gynecol Obstet Invest 2007;64:187-92.
2. El-Bana SM, El-Din AE, Isamil ZA. Fetal and maternal oxidative stress in normal and abnormal pregnancies. Ain Shams Med J 2001;52:421-31.
3. Orhan H, Onderoglu L, Yücel A, Sahin G. Circulating biomarkers of oxidative stress in complicated pregnancies. Arch Gynecol Obstet 2003;267:189-95.
4. Tastekin A, Ors R, Demircan B, Saricam Z, Ingee M, Akay, F. Oxidative stress in infants born to preeclamptic mothers. Ped Intl 2005;47: 658-62.
5. Karabulut AB, Kafkasli A, Burak F, Gozukara EM. Maternal and fetal plasma adenosine deaminase, xanthine oxidase and malondialdehyde levels in preeclampsia. Cell Biochem Func 2004;23:279-83.
6. Scaife AR, McNeill G, Campbell DM, Martindale S, Devereux G, Seaton A. Maternal intake of antioxidant vitamins in pregnancy in relation to maternal and fetal plasma levels at delivery. British J Nutr 2006;95:771-8.
7. Bolisetty S, Naidoo D, Lui K, Koh THHG, Watson D, Montgomery R, Whitehall J. Postnatal changes in maternal and neonatal plasma vitamins and the influence of smoking. Arch Dis Child Fetal Neonatal Ed 2002;86:F36-F40.
8. Kanishtha A, Arun D, Nanak P, Onkar K. Factors affecting serum vitamin A levels in matched-cord pairs. Ind J Paed 2008;75:443-6.
9. Kristin B, Harsem Nina H, Staff Anne C. Oxidative Stress and Antioxidant Status in Fetal Circulation in Preeclampsia. Ped Res 2006; 60:560-4.
10. Bowen RS, Moodley J, Dutton MF, Theron AJ. Oxidative stress in preeclampsia. Acta Obstetricia et Gynecologica Sacandinavica 2001;80:719-25.
11. Kim YH, Kim CH, Cho MK, Kim KM, Lee SY, Ahn BW, et al. Total peroxyl radical-trapping ability and anti-oxidant vitamins of the umbilical venous plasma and the placenta in pre-eclampsia. J Obstet Gynaecol Res 2006;32:32-41.
12. Bayadasa G, Karatasb F, Gursuc MF, Bozkurtd HA, Ilhanc N, Yasara A, Canatanc H. Antioxidant vitamin levels in term and preterm infants and their relation to maternal vitamin status. Arch Med Res 2002;33:276-80.
13. Noyan T, Güler A, Sekeroğlu MR, Kamaci M. Serum advanced oxidation protein products, myeloperoxidase and ascorbic acid in pre-eclampsia and eclampsia. Aust N Z J Obstet Gynaecol 2006;46:486-91.
14. Mutlu Turkoğlu U, Ademoğlu E, Ibrahimoğlu L, Aykac Toker G, Uysal M. Imbalance between lipid peroxidation and antioxidant status in preeclampsia. Gynecol Obstet Invest 1998;46:37-40.
15. Chappell LC, Seed PT, Kelly FJ, Briley A, Hunt BJ, Charnock-Jones DS, et al. Vitamin C and E supplementation in women at risk of preeclampsia is associated with changes in indices of oxidative stress and placental function. Am J Obstet Gynecol 2002;187:777-84.
16. Suhail M, Faizul-Suhail M, Hina K. Alterations in antioxidant and pro-oxidant balance in preeclampsia. Impact on Erythrocyte Osmotic fragility. Biochemia Medica 2008;18:331–41.
17. Suhail M, Faizul-Suhail M, Hina K. Role of Vitamins C and E in regulating Antioxidant and Pro-oxidant Markers in Preeclampsia. J Clin Biochem Nutr 2008;43:210-20.
18. Jain SK, McVie R, Duett J, Herbst JJ. Erythrocyte membrane lipid peroxidation and glycosylated hemoglobin in diabetes. Diabetes 1989;38: 1539-43.
19. Jagota SK, Dani HM. A new colorimetric technique for the estimation of vitamin C using Folin phenol reagent. Anal Biochem 1982;127:178-82.
20. Sobel AE, Snow SD. The estimation of serum vitamin A with activated glycerol dichlorohydrin. J Biol Chem 1947;171:617-32.
21. Fabianek J, Defilippi J, Rickards T, Herp A. Micromethod for Tocopherol Determination in Blood Serum. Clin Chem 1968;14:456-62.
22. Wang Y, Walsh SW. Placental mitochondria as a source of oxidative stress in pre-eclampsia. Placenta 1998;19:581-6.
23. Lowe DT. Nitric oxide dysfunction in the pathophysiology of preeclampsia. Nitric Oxide 2000;4,441-58.
24. Aydin A, Benian A, Modazli R, Uloda S, Uzun H, Kaya S. Plasma malondialdehyde, superoxide dismutase, sE-selectin, fibronectin, endothelin-1 and nitric oxide levels in women with preeclampsia. Eur J Obstet Gynecol Reprod Biol 2004;113:21-5.
25. Niki E, Saito T, Kawakami A, Kamiya Y. Inhibition of oxidation of methyl linoleate in solution by vitamin E and vitamin C. J Biol Chem 1984;259:4177-82.
26. Vanderlelie J, Venardos K, Clifton VL, Gude NM, Clarke FM, Perkins AV. Increased biological oxidation and reduced anti-oxidant enzyme activity in pre-eclamptic placentae. Placenta 2005;26:53-8.
27. Walsh SW, Wang Y. Deficient glutathione peroxidase activity in preeclampsia is associated with increased placental production of thromboxane and lipid peroxides. Am J Obstet Gynecol 1993;169:1456-61.
28. Schiff E, Friedman SA, Stampfer M, Kao L, Barrett PH, Sibai BM. Dietary consumption and plasma concentrations of vitamin E in pregnancies complicated by preeclampsia. Am J Obstet Gynecol 1996; 175:1024–8.
29. Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol 2005;3:28.