Increased IgA in a subject with positive anti-thyroid autoantibodies - a case report
Slavica Dodig
[*]
[1]
Renata Zrinski Topić
[1]
Jadranka Demirović
[2]
Jadranka Živčić
[1]
Introduction
Subclinical thyroid dysfunction is defined as an abnormal serum concentration of thyroid-stimulating hormone, TSH, when serum free thyroxine (T4) concentration is within its reference range. Subjects with subclinical disease have few or no definitive clinical signs or symptoms of thyroid disorder (1). Consequently, subclinical thyroid disease is a laboratory diagnosis.
Increased concentrations of antithyroid autoantibodies are found in inflammatory disaeses as well as in thyroid autoimmune disorders (2). These autoantibodies are directed against thyroid-specific molecules for the production of thyroid hormones, i. e. thyroperoxidase (TPO) and thyroglobulin (Tg). False positive and false negative interferences in quantitative immunochemical methods are possible in turbidimetric, nephelometric, but mostly in saturating immunoassays (3,4). It is known that the presence of serum anti-Tg antibodies has the potential to interfere with Tg measurement (5), especially in saturating immunoassays (6). Haroun M. et al described false positive results for IgA in 35 patients with anti-thyroid autoantibodies using ELISA test (7). These authors demonstrated that the problem in their immunoassay has been caused by the goat anti-IgA antibodies used in the ELISA.
The aim of this case report is to present false positive IgA in a female with subclinical thyroid dysfunction. Results throughout 13 months of follow-up are presented.
Case report
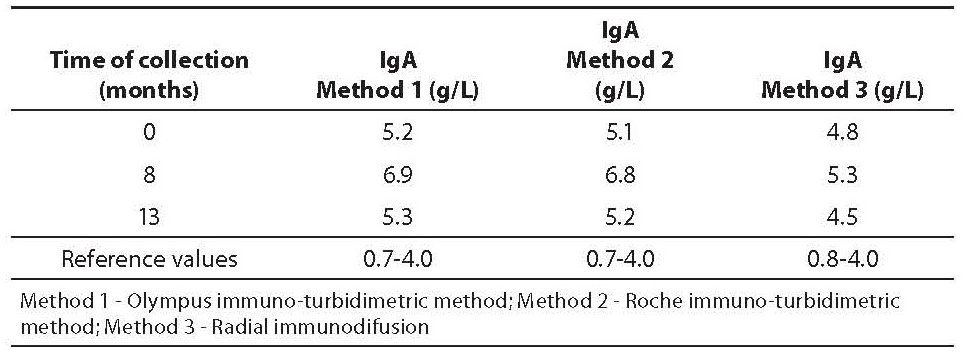
A 58-year-old, clinically healthy female with increased concentrations of anti-thyroglobulin (anti-Tg) autoantibodies is presented. In 1986, the patient underwent partial thyroidectomy because of the finding of benign thyroid nodules. Periodical serum thyroid function tests gave normal results until 2007. In August 2007 thyroid function tests showed normal values of T3 (1.5 nmol/L), T4 (119 nmol/L), and TSH (4.71 mIU/L), and increased values of thyroid peroxidase autoantibodies (anti-TPO, 12.2 kIU/L), anti-Tg autoantibodies (86.4 kIU/L) and increased IgA (5.24 g/L, determined by immuno-turbidimetric method; reference values: 0.7-4.0 g/L) (Table 1). The patient underwent treatment with single daily dose of 25 μg of levothyroxine, and all blood tests were performed on two occasions, i.e. after 8 (March 2008) and 13 (September 2008) months. The elevated initial levels of anti-TPO stabilized with the administration of levothyroxine (8.7 and 9.3 kIU/L after 8 and 13 months, respectively; reference values: < 12 kIU/L), while anti-Tg remain increased (58.7 and 54.2 kIU/L after 8 and 13 months, respectively; reference values: < 34 kIU/L). During that period, inflammatory parameters, i.e. erythrocyte sedimentation rate, leukocyte count, high sensitive C-reactive protein, IgG and IgM, were within reference intervals (data not presented). However, concentration of IgA was increased throughout 13 months of follow-up (Table 1). The levels of thyroid hormones and antibodies in the serum samples were measured on AxSYM Immunoassay Instrument System, using microparticle enzime-immunoassay (Abbott Laboratories, USA); IgA was determined by immuno-turbidimetric method (Method 1), with reagents containing goat anti-IgA antibodies (Olympus, Hamburg, Germany; Olympus AU 400 analyser). During of follow-up period of 13 months the subject was clinically healthy, free from any signs and symptoms of neither acute nor chronic disease. Since there was no clear clinical reason for increased IgA concentration, two additional methods were used to verify IgA values: 1. immuno-turbidimetric method, with reagents containing goat anti-IgA antibodies (Roche Diagnostics GmbH, Mannheim, Germany; Hitachi 912 analyzer) (Method 2), and 2. radial immunodiffusion, RID, with agar containing goat anti-IgA antibodies (Institute of Immunology, Zagreb, Croatia), (Method 3). The mean intra-assay coefficient of variation for all assays was < 10%.
Table 1. IgA concentration with three methods
Discussion
In this case report a subject with subclinical thyroid disease is presented. The earliest stage of thyroid dysfunction was confirmed by initial serum concentration of TSH (higher than 2.5 but less than 4.5 mIU/L) and increased concentration of anti TPO and anti-Tg autoantibodies. Such findings may identify subjects suspected for an early stage of hypothyroidism or Hashimoto thyroiditis (1). Positive anti-TPO antibodies identify an autoimmune etiology for thyroid disorder. The elevated initial levels of anti-TPO stabilized with the administration of levothyroxine, while anti-Tg remained increased. Usually, prolonged increase of IgA concentration might indicate that IgA is activated in protection of the mucous membranes. However, neither acute, nor chronic disease was documented in a female with subclinical thyroid disease. Since nonspecific inflammatory parameters were not indicative of severe inflammation, increased concentration of IgA seemed confusing. So, a possible interference in determination of IgA could be assumed to occur. According to the investigation of Haroun et al (6,7), patients with autoimmune thyroid diseases may have increased concentration of IgA (5.01 g/L) in comparison to healthy individuals (2.75 g/L).
In the present case report IgA, determined by immuno-turbidimetric method, ranged from 5.24-6.96 g/L (using Olympus immunoassay) and 5.1-6.8 g/L (using Roche immunoassay) throughout 13 months of follow-up. Values obtained by RID were lower (ranged from 4.3-5.3 g/L) than the values obtained by immuno-turbidimetric assays, especially in samples obtained after 8 and 13 months (difference greater than intra-assay coefficient of variation). However, all IgA values exceeded upper reference limits. It remains unclear whether the interferences typical for immunoassays have caused false increased IgA or not. False positive results in IgA could be a result of both, interferences of goat anti-IgA antibodies from reagents, and cross-reactivity with endogenous anti-Tg autoantibodies in serum, respectively.
We are aware that this case has some limitations, such as a determination of IgA without purification from the interfering anti-goat immunoglobulin antibodies, without use of blocking agent. To clarify the cause of possible false positive IgA values, future studies should performed in larger number of subjects with increased anti-Tg antibodies. Validation of agents that are best for reducing the effect of cross-reacting endogenous antibody-structured substances should be applied.
Acknowledgements
The authors wish to thank PhD. Anđa Treščec, Institute of Immunology, Zagreb, Croatia for carrying out the radial immunodifusion determinations and mag. med. biochem. Vlasta Zovko, Jordanovac Clinical Hospital, Zagreb, Croatia, for carryng out Roche immuno-turbidimetric assay.
Notes
Potential conflict of interest
None declared.
References
1. Surks MI, Ortiz E, Daniels GH, Sawin CT, Col NF, Cobin RH, et al. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA 2004;291:228-38.
2. Sinclair D. Clinical and laboratory aspects of thyroid autoantibodies. Ann Clin Biochem 2006;43:173-83.
3. Tate J, Ward G, Interferences in immunoassay. Clin Biochem Rev 2004;25:105-20.
4. Dodig S. Interferences in quantitative immunochemical methods. Biochem Med 2009;19:50-62.
5. Madureira D, Prazeres S, Pedro MS, Pereira T, Font AP, Bugalho MJ. In vitro assays to test the interference of anti-thyroglobulin antibodies on thyroglobulin measurement. Endocrine 2008;33:40-4.
6. Spencer CA, Bergoglio LM, Kazarosyan M, Fatemi S, LoPresti JS. Clinical impact of thyroglobulin (Tg) and Tg autoantibody method differences on the management of patients with differentiated thyroid carcinomas. J Clin Endocrinol Metab 2005;90:5566-5575.
7. Haroun M, El-Masry MH. Antibodies reacting with human immunoglobulin in sera from autoimmune thyroid disease patients as a risk factor for false positive results in IgA assessment. Centr Eur J Immunol 2008;33:208-12.