When I became a journal editor, back in the 90ties, I was not really aware that I was embarking into a profession very different from research and teaching. We usually think that one is qualified to be a journal editor just because he or she is an expert in a given research area. However, the scientific publishing enterprise in medicine is today very technologically advanced and very regulated, from the registration of clinical trials as a requirement for manuscript submission (1,2) to the common conflict of interest disclosure forms (3).
Promoting good editorial practices
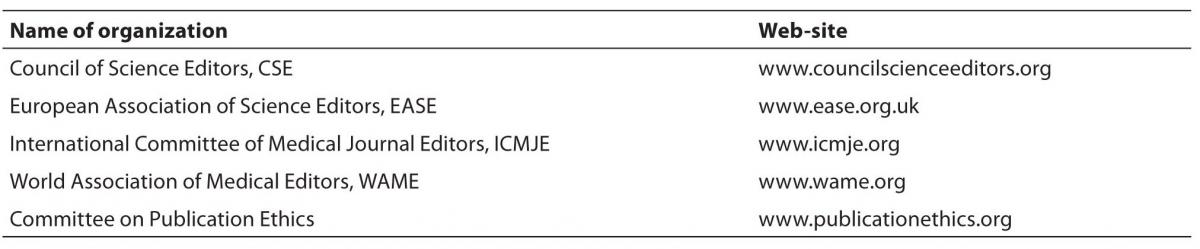
For an editor of small scholarly, usually a specialty journal in a small research community, who mostly works in isolation from other journal editors (4,5), it is not easy to quickly master all skills of the publishing profession, from manuscript tracking and review process to bibliographical and citation indexes and electronic publishing (6). This is the reason why editors have created associations that provide training, assistance and expertise for journal editors (Table 1). These organizations have regular meetings, educational activities, guidelines and expert counsel for their members. Some of them gather editors from all areas of research, and some have specialized in medicine, such as International Committee of Medical Journal Editors (ICMJE), the organization that established the so-called “Vancouver” style of reference writing (such as is used in the Biochemia Medica and most other health research journals) and the Uniform Requirements for Manuscripts Submitted to Biomedical Journals (
www.icmje.org).
Table 1. Associations and organizations for journal editors
Guidelines and codes of practices from editorial organizations deal not only with the actual editorial work, such as peer review and publishing, but also with ethical issues in editorial work. Responsible publishing and integrity of the published record are particularly important in health-related research because publications in scientific journals may have profound effect on health, potentially providing salvation to many lives or significantly improving the quality of life of individuals and populations (7). This is the reason why medical editors in particular, and science editors in general, are not only the gatekeepers of science quality in their journals, but also gatekeepers or research and publication integrity.
In the present system of scientific research and publication, which is based on mostly poorly founded trust, journals are often the first places where a whistle is blown about research misconduct because journal article is the best publicly visible documentation of research activity (8). This is the reason why journal editors are so particular about the integrity of the research record they publish (9). They are also aware that preserving this integrity is a difficult task, as well as often dangerous for the editor, especially if he or she works in a small scientific community (10).
Research integrity in small scientific communities
Small scientific communities are not burdened only by the vicious circle of scientific inadequacy, where poor research begets poor publications (11), but also by the vicious circle of poor research integrity. Lack of merit based on excellence in small communities creates an environment of “research corruption” (Figure 1): In a vicious circle, which supports weak science and inadequate researchers, small journals can often have a negative influence on local scientific community – its criteria, teaching, communication and scientific output. Weak publishing criteria result in low quality publications, but they are still recognized and valued as acceptable scientific research and counted for research and academic advancement. By publishing mostly in local journals, the researchers fail to perceive the incentives for improvement and for testing their research in the global community. Finally, they become a powerful obstacle for introducing international criteria in research because they promote weak criteria. Such publication practices foster research corruption and not research integrity. Once poor publications in local journals become the key criterion of (local) scientific and academic recognition, these journals become important to authors, journal editors, publishers and their owners. They build up a closed system of private interests, academic and political influence, nepotism, and no responsibility for the public interest.
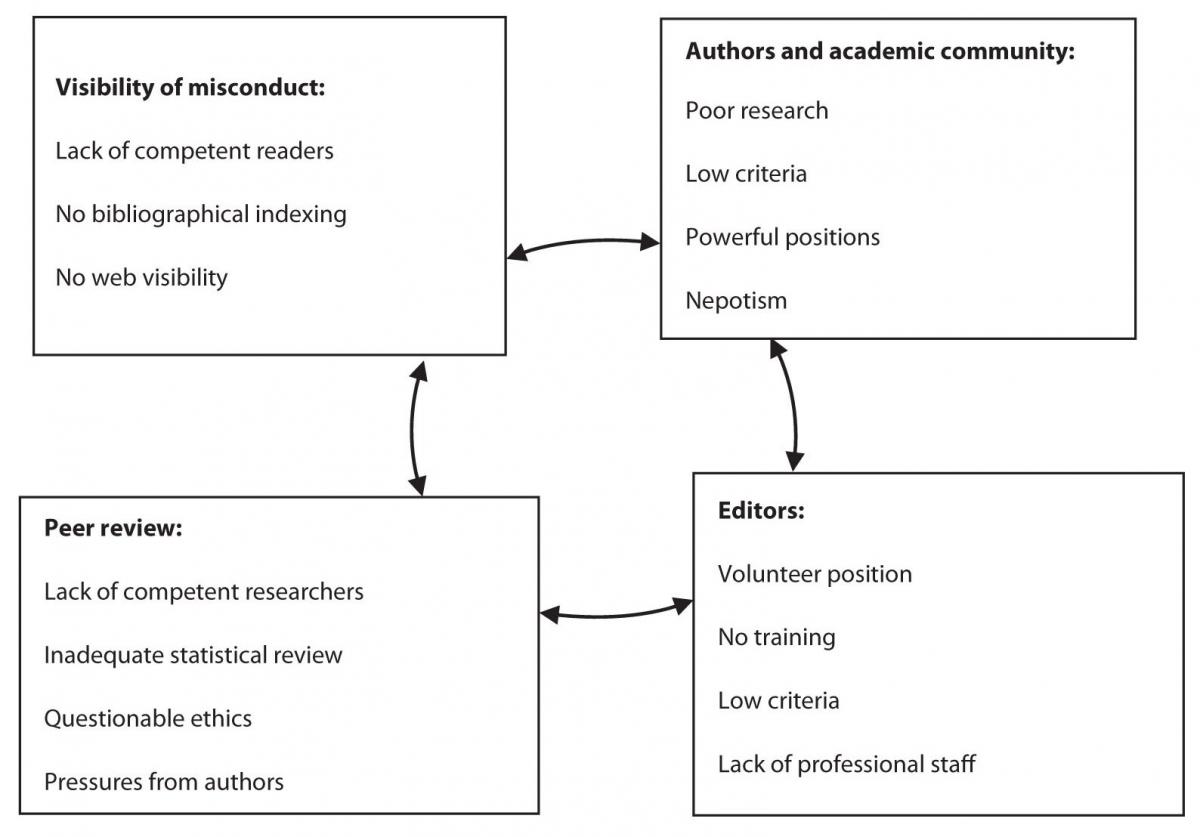
Figure 1. Integrity threat to small journals: vicious circle of publication corruption.
The problem of small academic communities rarely involves serious research fraud, such as falsification, fabrication and plagiarism, but rather high prevalence of irresponsible research practices, such as self plagiarism, redundant publications, salami publications, selective data publication, changing outcome measures, and improper statistics (7,12-14). Authorship misuse is also common, from gift to guest authors and from ghost authors to the author order on the article byline (15).
Detecting and preventing publication misconduct
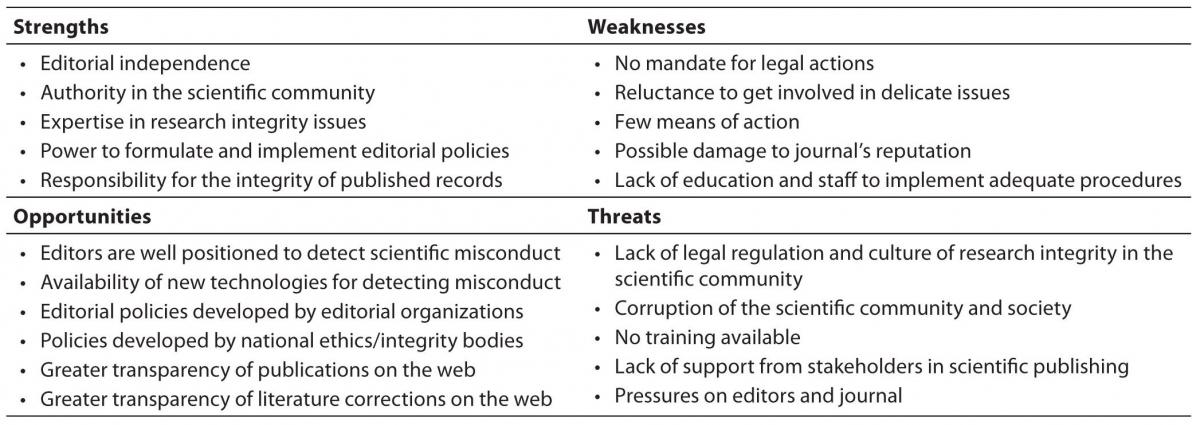
Editors in small journals may have problems in ensuring the full integrity of the articles published in their journals, mostly because of their own weaknesses as well as external threats to the integrity of the editorial work (15). In our recent SWOT (strengths-weaknesses-opportunities-threats) analysis of editors’ role in fostering responsible research publishing, we identified a number of problems but also several solutions for the editors as gatekeepers of research integrity (15). In small journals, the weaknesses and external threats to the job often outweigh their strengths and opportunities provided by the global editorial community (Table 2).
Perhaps the greatest strength of journal editors in preventing misconduct and fostering research integrity is the authority they have in their own scientific community. As editors of scholarly journals, they are usually at a high academic position, where they can influence not only the authors of the journal but also the wider academic community. Together with other strengths, such as editorial independence, expertise in research integrity issues, power to formulate and implement editorial policies, and the responsibility for the integrity of the published record, editors may be the key figures in increasing the level of research integrity in the scientific community. Editors should also be ready to face and work on their own weaknesses, such as reluctance to get involved in delicate issues, lack of clear mandate for action, possible legal problems and damage to the journal’s reputation, as well as shortage of staff to implement adequate procedures.
The opportunities for editors are provided by the larger editorial community and well-developed guidelines for good publishing practice. (Table 1 and Table 2). Particularly useful for editors are the guidelines from the Committee on Publication Ethics (COPE), the largest editorial organization dealing with actual ethical problems of journal editors (7). COPE has developed ethics flow charts – algorithms for editors to follow when they have an ethical problem in their journals. They are available at the COPE web-site (
http://publicationethics.org/flowcharwts) and are also useful for authors because they can learn about journal procedures related to ethical issues and about their own responsibilities and rights in the publication process. COPE flow-charts have also been translated into different languages, including Croatian. The Croatian translation is available at the web-site of the Croatian Ministry of Science, Education and Sports (
http://public.mzos.hr/Default.aspx?art=7966).
Table 2. SWOT analysis of the editors’ role in fostering responsible publishing of research (summarized from ref. 12).
Forensic tools for editors
Editors have several tools to detect scientific fraud. One of them, the Déjà vu database of very similar abstract texts from Medline/PubMed bibliographical database (
http://spore.vbi.vt.edu/dejavu/), is available to the general public, so that both editors and authors can check if there are duplicated or even plagiarized publications in their journals or bibliographies, respectively. Many journal editors, including us at the Croatian Medical Journal, have been contacted by the creators of the Déjà vu database about potential duplicate publications. Some of them were acceptable secondary publications (republication of the article in a different language or for different audience, with clear reference to the original publication), but some cases turned out to be plagiarized articles and need editorial decision of retracting the article. This is not something that editors like to do, but it is often the only way for editors to ensure the integrity of the research record published in their journals (10). COPE has recently published the guidance for editors about article retraction (16), to help them make the right decision, especially when they are not sure by whom or when an article has to be retracted.
The problem with the Déjà vu database is that includes only similarities in the text of the abstracts, but not the whole articles. Recently, new plagiarism detection software was developed for scientific publications. CrossCheck (
http://www.crossref.org/crosscheck.html) uses a database of scientific content from different publishers to check for text similarities. The Croatian Medical Journal is a part of the CrossCheck database and currently collaborates with the research group from the University of Rijeka School of Medicine, which works on a COPE research project to look into the detection possibilities of CrossCheck for plagiarism in small scientific journals.
Editors can also prevent publication of grossly manipulated images in scientific articles. According to the current policies on image manipulations, “no specific feature within an image may be enhanced, obscured, moved, removed, or introduced. Adjustments of brightness, contrast, or color balance are acceptable if they are applied to the whole image and as long as they do not obscure or eliminate any information present in the original. Nonlinear adjustments must be disclosed in the figure legend.” (17). Images can be easily manipulated in Photoshop, as every (at least young) researcher knows, but these manipulations are as easily detected by editors with minimal skills in the same software. Editors can also use special “forensic droplets”, developed by the Office for Research Integrity (ORI) of the USA – these are desktop applications for Adobe Photoshop which automatically examine features of a digital image and are available for free from the ORI web page
http://ori.dhhs.gov/tools/data_imaging.shtml.
Transparency of clinical trials
Editors of medical and health journals have yet another important task in ensuring the integrity of the research they publish. From 2004, they have to pay special attention to the registration of clinical trials as a precondition for manuscript submission to the journal (1,2). The ICMJE requirement for trial registration has been accepted by the World Health Organization, which developed a special portal for trial registries – International Clinical Trials Registry Platform (
http://www.who.int/ictrp/en/). The newest revision of the World Medical Association Helsinki Declaration on Ethical Principles for Medical Research Involving Human Subjects from 2008 (
http://www.wma.net/en/30publications/10policies/b3/index.html) also followed the editors’ initiative and introduced the new requirement for trial registration: “19. Every clinical trial must be registered in a publicly accessible database before recruitment of the first subject.” With the most recent directives of the European Commission for opening the EudraCT database, European journals and their editors can make a significant contribution to the process of increasing the transparency of clinical research for the benefit of the public. In Croatia, where there the legal requirement to make public all approved clinical trial is in power since 2007 but has not been implemented so far, a research group from the University of Split School of Medicine received the information technology grant from the Croatian Ministry of Science, Education and Sports and established a national register of trials in Croatian,
www.RegPok.hr, in collaboration with the largest trials database
www.ClinicalTrials.gov of the National Library of Medicine in the USA.
Instead of a conclusion
Despite all weaknesses and possible threats to their work, journal editors are not just passive eyewitnesses of scientific misconduct discovered after publication in their journals but can be active gatekeepers of responsible conduct and reporting of research. Just as it is easy today for a willing researchers to use modern technologies to fabricate, falsify and duplicate their data or publications, it is as easy for other stakeholders, including editors, to discover such misconduct. Journal editors have the strength of their editorial community in fulfilling this role. To be good gatekeepers, they have to learn about their responsibilities and rights as journal editors, to stay informed of the new developments, and, perhaps most importantly, teach their scientific community about responsible conduct of research.
Notes
Potential conflict of interest
None declared.
References
1. Laine C, Horton R, DeAngelis CD, Drazen JM, Frizelle FA, Godlee F, et al. Clinical trial registration: looking back and moving ahead. Croat Med J 2007;48:289-91.
2. Marušić A. Registration of clinical trials still moving ahead – September 2008 update to Uniform Requirements for Manuscripts Submitted to Biomedical Journals. Croat Med J 2008;49:582-5.
3. Drazen JM, Van Der Weyden MB, Sahni P, Rosenberg J, Marušić A, Laine C, et al. Uniform format for disclosure of competing interests in ICMJE journals. Croat Med J 2009;50:427-8.
4. Marušić M, Marušić A. Good editorial practice: Editors as educators. Croat Med J 2001;42:113-20.
5. Marušić A, Marušić M. Biochemia Medica – how to grow into a recognizable scientific journal? Biochem Med 2006;16:5-7.
6. Marušić A, Marušić M. Double life of medical journals: Dr Paper and Mr Web. Croat Med J 2006;47:4-6.
7. Marušić A. Approaches to the detection of research misconduct – The role of the peer review process. In: Wells F, Farthing M, eds. Fraud and Misconduct in Biomedical Research. London: The Royal Society of Medicine Press, 2008.
8. Marušić A, Marušić M. Killing the messenger: should scientific journals be responsible for policing scientific fraud? MJA. 2006;184:596-7.
9. Gollogly L, Momen H. Ethical dilemmas in scientific publication: pitfalls and solutions for editors. Rev Saude Publica 2006;40:24-9.
10. Marušić M, Marušić A. Threats to the integrity of the Croatian Medical Journal. Croat Med J 2007;48:779-85.
11. Marušić A, Marušić M. Small scientific journals from small countries: breaking from a vicious circle of inadequacy. Croat Med J 1999;40:508-14.
12. Katavić V. Five-year report of Croatian Medical Journal’s Research Integrity Editor – policy, policing, or policing policy. Croat Med J 2006;47:220-7.
13. Petrovečki M. The role of statistical reviewer in biomedical scientific journal. Biochem Med 2009;19:223-30.
14. Šimundić AM, Nikolac N. Statistical errors in manuscripts submitted to Biochemia Medica journal. Biochem Med 2009;19:294-300.
15. Marušić A, Katavić V, Marušić M. Role of editors and journals in detecting and preventing scientific misconduct: strengths, weaknesses, opportunities, and threats. Med Law 2007;26:545-66.
16. Wager E, Barbour V, Yentis S, Kleinert S, on behalf of COPE Council. Retractions: Guidance from the Committee on Publication Ethics (COPE). Croat Med J 2009;50:532-5.
17. Rossner M, Yamada KM. What’s in a picture? The temptation of image manipulation. J Cell Biol 2004;166:11-15.