Introduction
Neuroendocrine control of appetite
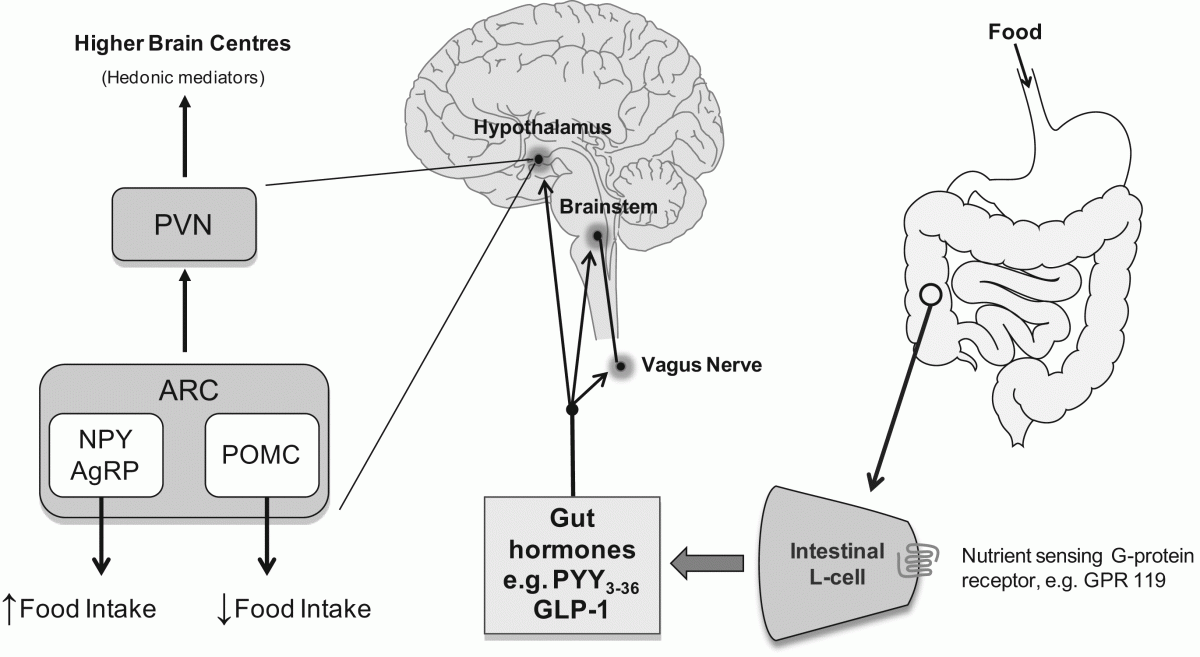
ARC - arcuate nucleus; AgRP - agouti related peptide; GLP-1 - glucagon like peptide-1; NPY - neuropeptide Y; POMC – propiomelanocortin; PVN - paraventricular nucleus; PYY - peptide YY.
The arcuate nucleus (ARC) of the hypothalamus is believed to play a crucial role in the regulation of food intake and energy homeostasis. The ARC contains two populations of neurons with opposing effects on food intake (5). Orexigenic neurons (i.e. those stimulating appetite) express neuropeptide Y (NPY) and Agouti-related protein (AgRP) (6-8). Whilst anorexigenic neurons (i.e. those inhibiting ap-petite) in the ARC express alpha-melanocyte-stimulating hormone (alpha-MSH) derived from pro-opiomelanocortin (POMC), and cocaine- and amphetamine-regulated transcript (CART) (9).
The ARC is adjacent to the median eminence, a ‘circumventricular organ’ with fenestrated capillaries and hence an incomplete blood-brain barrier (10). Circulating hormones are able to pass across the median eminence and influence the activity of the ARC neurons directly. Gut hormones are released from the gastrointestinal tract on a meal to meal basis and signal short term nutrient availability to the ARC. Other circulating factors such as insulin and leptin (a circulating peptide released from adipose tissue) relay information about long-term energy stores and adiposity (11). Thus the ARC has been described as a conduit through which the body can balance its energy requirements to maintain weight.
Additionally short term availability of nutrients is signalled by gastrointestinal vagal afferents. Follow-ing a meal the vagus is activated by both mechanoreceptors and chemoreceptors. The resultant neu-ral signals converge in the nucleus of the tractus solitarius (NTS) within the brainstem. These signal are then fed forward Neuronal from the NTS to the hypothalamus. Circulating factors such as gut hormones are also thought to act at the NTS, which like the ARC is adjacent to a circumventricular organ, the ‘area postrema’ (AP). For example, ablation of both the AP and another circumventricular organ, the subfornical organ (SFO), has been shown to delay the anorectic action of the gut hormone peptide tyrosine tyrosine (PYY) (12). Gut hormones also alter the activity of the ascending vagal pathways from the gut to the brainstem (13).
Hence, the hypothalamic ARC orexigenic and anorexigenic neurons are influenced by numerous neu-ral and hormonal inputs. These ARC neurons in turn project to a number of extra-hypothalamic and intra-hypothalamic regions, including in particular the hypothalamic paraventricular nucleus (PVN), where some of the important efferent pathways regulating energy expenditure arise.
Enteroendocrine cells of the gastrointestinal tract
Fatty acids derived from digestion of dietary fats appear to be sensed via separate mechanisms. The short-chain fatty acid receptors GPR43 and GPR41 are expressed in PYY-containing enteroendocrine L cells (18,19). Short chain fatty acids have been shown to increase both PYY and GLP-1 secretion in rats when delivered directly into the colon (20,21). GPR119 is another G protein coupled receptor found in intestinal endocrine cells as well as pancreatic beta cells (22). Administration of oleoyletha-nolamide (OEA), an endogenous long chain fatty acid derivative, and other GPR119 agonists in-creases GLP-1 secretion, both in vitro and in vivo in rodents (22,23).
The enteroendocrine L cells therefore have the capacity to integrate complex nutrient sensing in the gut and to respond appropriately by releasing gut hormones. In addition to chemical stimulation, the endocrine cells of the gut also respond to neural and physical stimulation of the cell by releasing pep-tide containing granules at the basolateral side of the cell. These peptides can have an endocrine role, a local paracrine role, and/or activate receptors present on nerves innervating the GI mucosa (24).
Gut hormones regulating food intake
Gut hormones are believed to contribute to the short-term feelings of satiety and hunger (28). These peptides are thought to reduce food intake by decreasing hypothalamic orexigenic signalling and in-creasing anorectic signalling (13,29). These peptides also mediate inhibitory feedback mechanisms on intestinal transit, contributing to prolonged gastric distension, and increased satiety between meals (30,31). These combined CNS effects and ‘intestinal brake’ mechanisms facilitate the control of food intake and postprandial transit through the gastrointestinal tract and thereby the immediate availability of energy. Below the focus of the review will concentrate on 5 of the most studied gut hormones which have been shown to control food intake and body weight and which are being actively pursued as anti-obesity targets.
Peptide tyrosine tyrosine (PYY)
Low levels of PYY are detected in enteroendocrine cells in the stomach, and levels increase distally along the small and large intestine, reaching their highest levels in cells in the colon and rectum (26). Endogenous circulating concentrations of PYY are lowest in the fasting state, and rise post-prandially in proportion to caloric intake (26). Plasma levels of PYY rise within 30 minutes of a meal, and in hu-mans, circulating levels plateau at 1-2 hours post-prandially, remaining elevated for up to 6 hours (38). Protein rich meals cause the greatest increase in PYY levels compared to other macronutrients (39,40). Peripheral administration of PYY3-36 reduces food intake and weight gain in rodents (29,41-43). Intravenous administration of PYY inhibits food intake in humans and unlike leptin is equally ef-fective in normal and obese subjects (44).
The anorectic effects of PYY3-36 appear to be mediated centrally via the ARC, as peripheral admini-stration of PYY3–36 increases c-fos expression in this hypothalamic nucleus (29). Peripheral admini-stration has been reported to decrease expression and release of NPY whilst activating POMC neu-rons (29). However, others have reported PYY3–36 inhibits POMC neurons via postsynaptic Y2R (45). Moreover, POMC knockout mice maintain their acute anorectic response to peripherally administered PYY3–36, suggesting that POMC is not critical to the inhibitory effects of PYY3–36 on feeding (46).
A vagal brainstem mediated pathway may also be involved since PYY is expressed by the neurones of the myenteric plexus and the Y2R receptor is expressed by the vagus nerve (47). Furthermore the anorectic effect of PYY3–36 on both food intake (47,48), and ARC activation of feeding neurons, are abolished following bilateral sub-diaphragmatic total truncal vagotomy or following transection of the brainstem–hypothalamic pathway in rodents (48).
Interestingly, it has recently been shown that acute effects of gastrointestinal bypass surgery on body weight are lost in PyyKO mice (49), and that wild-type mice losing weight after gastrointestinal bypass surgery exhibit increased colonic Pyy expression and circulating fasting PYY levels (49). Suggesting PYY plays a key role in mediating the early weight loss that occurs following gastrointestinal bypass surgery.
The effects of PYY3-36 on satiety and central control of appetite are clear. Most are mediated via an-orectic neuronal populations in the ARC, but vagal/brainstem-mediated pathways and peripheral ef-fects of PYY on gastric emptying and intestinal motility may also play a part. High plasma concentra-tions of PYY result in nausea, but the importance of PYY3-36 at physiological levels in the regulation of energy intake make it a prime focus for new obesity therapies, targeted either at PYY itself, or against the Y2 receptor.
Glucagon-like peptide-1 (GLP-1)
Like PYY, GLP-1 has been shown to act centrally at hypothalamic nuclei known to be implicated in the control of appetite including the ARC, PVN and supraoptic nucleus (57). Both acute peripheral and central administration of GLP-1 reduce food intake in rats (58,59) and chronic administration of GLP-1 reduces weight gain (55). The intravenous administration of GLP-1 to normal and obese hu-mans decreases food intake in a dose dependent manner (60) as well as reducing gastric emptying (61,62). These effects are thought to be mediated through vagal and brainstem pathways since pe-ripheral administration of GLP-1 activates neurons within the brainstem in rats (63). Furthermore, this increase in neuronal activity, and the anorectic effects of GLP-1, are abolished following vagotomy in rodents (48,63). More recently, functional magnetic resonance imaging (fMRI) has confirmed the acti-vation of the VMH and PVN following peripheral administration of GLP-1 (64).
GLP-1 is rapidly degraded in the circulation by DPP-IV, making native GLP-1 unsuitable for therapeu-tic use. Longer acting GLP-1 mimetics have been developed (65). Exendin-4 is a naturally occurring GLP-1 mimetic isolated from the venom of Heloderma suspectum, a lizard native to several south-western American states (66). A truncated form of this peptide, exendin 9–39, acts as a competitive antagonist at the GLP-1 receptor. Acute intracerebroventricular administration of exendin 9–39 in-creases food intake and chronic administration increases body weight in rats (55,59). Suggesting en-dogenous peripheral GLP-1 may physiologically reduce appetite and food intake. However, GLP-1 receptor knockout mice do not have altered food intake or body weight (67). This may be because developmental changes compensate for the lack of GLP-1 signalling, or may reflect that GLP-1 has a more important physiological role in controlling blood glucose than in regulating food intake.
The discovery of exendin-4 has led to the development of a synthetic version, exenatide. Exenatide has a much longer in vivo half-life than native GLP-1, stimulates insulin release, suppresses glucagon and lowers blood glucose. It is the first incretin mimetic approved for the treatment of type 2 diabetes (68). Exenatide has also been shown to reduce body weight in treated diabetics in phase III clinical trials (69-71). The weight loss associated with exenatide is considered a significant advantage as many anti-diabetic treatments are commonly associated with weight gain. Nausea is a relatively common side effect of Exenatide treatment. However, it does not seem to be intrinsically linked to the effects on appetite (3). Whilst GLP-1 has been developed as a treatment for diabetes due to its in-cretin properties, the observed effects of GLP-1 on satiety and weight loss are a valuable secondary effect. Indeed recent data suggests liraglutide may be useful for the treatment of obesity, causing sus-tained weight loss over 2 years but with a 50% rate of nausea and vomiting in the 3.0 mg/day group in the first year (72). The newest long acting analogues of GLP-1, exenatide-LAR (Amylin Pharmaceuti-cals, FDA approved January 2012), taspoglutide (Ipsen and Roche) and Albiglutide (GalxoSmith-Kline), have been shown to effectively control glucose and to reduce weight. These agents allow for less-frequent dosing schedules, improved glycemic control throughout the day, and improved treat-ment satisfaction compared to some available agents (73). It remains to be seen whether these drugs perform well enough in specific weight loss paradigms such that they could be used as anti-obesity agents.
Oxyntomodulin (OXM)
Although OXM has some agonist activity at the glucagon receptor, there is evidence that its anorectic effect is predominantly mediated via the GLP-1 receptor (75,81). The anorectic effects of OXM are abolished in GLP-1 receptor knockout mice (81) and in the presence of the GLP-1 receptor antagonist exendin 9-39 (76). OXM has a 50-fold lower affinity for the GLP-1 receptor than GLP-1 itself, but de-spite this, it reduces food intake with similar potency (75). Furthermore, although the administration of exendin 9-39 directly into the ARC blocks the anorectic effects of OXM, it does not block those of GLP-1 (76). Therefore, it is possible that OXM may act via an as yet unidentified receptor. Studies using manganese-enhanced magnetic resonance imaging MRI (MEMRI) has shown that intraperito-neal administration of OXM produces a distinct pattern of neuronal activation compared to GLP-1 (82), implying that these two hormones act via different hypothalamic pathways.
Glucagon
Glucagon mediates its effects via the glucagon receptor, a 7-transmembrane G-protein coupled re-ceptor which has a wide tissue distribution. It is expressed in the gut, adrenal glands, brain, heart, pancreas, spleen and in adipocytes, but is predominantly found in the liver and kidney (87).
As a potential treatment for obesity, glucagon has been shown to increase energy expenditure in rats, and also in humans during insulin deficiency (88). It also significantly reduces food intake, with a sub-jective reduction of appetite in man (89). Infusion of glucagon into the portal vein but not the inferior vena cava causes a reduction in meal size in rats (90).
Glucagon presents an interesting prospect in the treatment of obesity due to its effect on increasing energy expenditure, and increasing satiety. It has been demonstrated that the potentially unfavour-able effect on glucose tolerance due to glucagon’s actions on hepatic glycogenolysis and gluconeo-genesis is effectively counteracted by dual agonism at the glucagon and GLP-1 receptors (91,92). The data from these studies demonstrated highly effective weight loss in diet-induced obese mice whilst avoiding the hyperglycaemia that might be expected from agonism at the glucagon receptor.
Ghrelin
Ghrelin is the only orexigenic gut hormone (94), causing an increase in food intake and weight gain in rodents following both peripheral and central administration (95-97). Intravenous administration of ghrelin has also been shown to stimulate gastric acid secretion and motility in rats (98). In normal sub-jects, ghrelin levels are highest in the fasted state (99), and levels are chronically higher in people with weight loss due to anorexia nervosa or dietary reduction (100-102). In contrast to other gut hor-mones, plasma ghrelin levels decrease after meals (100,103) and are low in obese subjects (102). Ghrelin concentrations are also reduced after gastric bypass surgery, and this may contribute to weight loss in such patients (101).
Ghrelin receptors are found in the ARC of the hypothalamus suggesting a central mode of action. Consistent with this c-fos expression is increased in the ARC after peripheral administration of ghrelin (104) and ablation of the ARC blocks ghrelin induced food intake (105). When given centrally, ghrelin also stimulates c-fos expression in other nuclei known to be involved in appetite control including the PVN, dorsomedial nucleus, and lateral hypothalamus as well as in the AP and NTS in the brainstem (95). Ghrelin and its receptor are both expressed in vagal afferents in mice (106), and blockade of the gastric vagal afferent has been shown to abolish ghrelin-induced feeding, growth hormone secretion, and activation of NPY-producing and growth hormone-releasing hormone producing neurons in rats suggesting an additional mode of action (107).
Diet induced obesity is associated with a blunting of ghrelin’s orexigenic effect. There has therefore been recent interest in the interaction between the ghrelin system and macronutrients. High fat feed-ing has been shown to render NPY/AgRP neurones relatively ghrelin resistant (108), and diets high in fat have been shown to directly inhibit the hyperphagic effect of ghrelin (109,110). These data have significant implications for developing anti-obesity treatments targeting the ghrelin system and sug-gest success of these approaches could depend on the fat content of the diet the patient consumes. More recently, ghrelin has been shown to engage neurons in the ventral tegmental area of the brain and may provide a link between the gut and neuronal control of stress-induced eating of ‘comfort foods’ (111).
Other gut peptides
CCK is released post-prandially from the small intestine (3), and has also been shown to co-localise with PYY in L cells (112) Two types of CCK receptor have been identified in the CNS and peripheral tissues CCK1 and 2 (113). CCK is released post-prandially in response to saturated fat, long-chain fatty acids, amino acids and small peptides that would normally result from protein digestion (114,115). CCK release and signalling via the CCK-1 receptors in response to these long chain fatty acids mediates stimulation of PYY release and inhibition of ghrelin (an orexigenic gut hormone) in human subjects (116).
The effects of CCK on appetite are well documented. Peripheral administration of CCK in rodents results in a dose dependant reduction in food intake, decreasing both meal size and duration (117). CCK administration is also associated with an increase in postprandial satiety behaviours such as increased grooming and decreased locomotor activity (117). In humans, intravenous administration of physiological doses of CCK reduces food intake and increases the perception of fullness (118). Unfor-tunately, the therapeutic potential of CCK as a treatment for obesity is limited by nausea and tachy-phylaxis of the anorectic effects associated with chronic administration (119).
PP is an amidated 36-amino acid peptide and belongs to the ‘PP fold’ family of peptides. It is released post-prandially under vagal control by pancreatic islet PP cells (120-122). PP binds to all the mem-bers of the Y receptor family, but has the highest affinity for the Y4 receptor subtype (123). The effects of PP are likely to be mediated by both the hypothalamus and brainstem (124). PP is comparable to other anorectic intestinal peptides such as PYY, being secreted in proportion to caloric intake. Circu-lating levels rise after meals and remain elevated for up to 6 hours post-prandially (120). Intraperito-neal injection of PP acutely reduces food intake in fasted mice (124), an effect that remains apparent for 24 hours after injection. Furthermore, chronic administration of PP over 6 days in ob/ob mice sig-nificantly reduces body weight gain and improves glucose profile (124). Intravenous infusion of PP at doses that achieve normal post-prandial plasma concentrations reduces appetite in lean humans and inhibition of food intake persists for 24 hours after infusion (125). PP has also been shown to reduce food intake at lower infusion rates (126). Furthermore, pancreatic polypeptide has been shown to re-duce food intake in patients with obesity secondary to Prader-Willi syndrome (127). Additionally, PP has also been implicated in energy homeostasis, with exogenous administration of PP causing an increase in oxygen consumption (124), thus implying that part of the effect of PP on body weight may be due to increased energy expenditure. It has also been shown to increase spontaneous locomotor activity in mice (128). These data have lead to a concerted effort to develop long acting PP ana-logues, which have completed Phase I trials (129).
NT was first isolated from hypothalamic tissue, but is widely distributed throughout the central nervous system. However, the majority of NT is found within enteroendocrine cells of the GI tract (130). NT regulates a number of digestive processes, including gastrointestinal motility, and pancreatic and bil-iary secretion (131). It also has trophic effects on the pancreas and small intestine (132,133). Plasma levels of NT increase after a meal, with intraluminal fat being the most potent stimulus (134). Periph-eral administration of neurotensin decreases food intake and grooming behaviour in rats only at large doses (135). Therefore at physiological levels, neurotensin is unlikely to play a major role in appetite regulation. Although neurotensin acutely reduces food intake when administered centrally in rats or peripherally in mice, chronic administration to mice has no significant effect on food intake or body weight (136). The lack of chronic effects on body weight suggests that NT is unlikely to be useful as a treatment for obesity.
Intracerebroventricular injection of glucagon-like peptide-2 (GLP-2) into rats inhibits food intake. In contrast, GLP-2 administered peripherally does not inhibit food intake in rodents or humans (137,138). GLP-2 appears to play a more important physiological role as an intestinal growth factor (138).
Amylin is a peptide co-secreted with insulin by pancreatic beta cells. Injection of amylin or amylin ag-onist has been shown to reduce food intake in a number of species, including humans (139-143). The amylin receptor agonist pramlintide has been shown to cause weight loss in diabetic humans (141,143).
Vasoactive intestinal polypeptide (VIP) has been shown to reduce appetite, in addition to its well-characterized effects on the cardiovascular system and gastrointestinal motility and secretion. In-tracerebroventricular administration of VIP has been shown to cause a potent short-lived decrease in food intake and an increase in activity and energy expenditure in rats. Treatment of hypothalamic ex-plants with VIP stimulated the release of the anorexigenic peptide α-MSH (144). These studies sug-gest a possible endogenous role for VIP in the hypothalamic control of energy homeostasis.
Gut hormones and the treatment of obesity
The only obesity treatment that has been shown to confer long-term, sustained weight loss and a de-crease in overall mortality is bariatric surgical intervention (148,149). Several surgical procedures are available to achieve weight loss. Gastric banding restricts the amount of food that can be comfortably ingested and increases the satiating effect of food (150). A more efficient reduction in appetite and weight loss is seen with surgical procedures that involve gastrointestinal bypass, such as Roux-en-Y Gastric bypass (RYGB) (148,149,151). Weight loss is normally associated with reduced plasma levels of the adipocyte-derived anorectic hormone leptin, causing increased hunger (152). However, follow-ing RYGB, despite significant reductions in body weight and leptin levels, appetite is markedly re-duced (151). It has now been demonstrated that RYGB is more effective than either standard or in-tensive medical therapy in achieving glycaemic control and remission in patients with Type 2 diabetes (T2DM) (153,154). These seminal studies raise the question as to whether bariatric surgery could become a more important treatment for T2DM than medical therapy. Indeed, in a recent positional paper the International Diabetes Federation supported the selective use of various bariatric proce-dures for obese individuals with medically resistant T2DM (155). However, significant questions re-main, not least is how long do these effects last? But also include when is the best time for surgical intervention? Does bariatric surgery work for everyone? What are the surgical/risk benefits in moder-ately obese patients? This list is by no means exhaustive. Given RYGB requires major surgery, which has inherent risk and is expensive, there is considerable effort aimed at determining how RYGB and other surgeries induce sustained weight loss and resolution of T2DM.
Of particular interest has been the suggestion that RYGB ameliorates coexistent type 2 diabetes mel-litus before substantial weight loss has occurred and more rapidly than gastric banding. The differ-ences between gastric banding and RYGB may be due to alterations in the anorectic and incretin gut hormone profile that is seen following RYGB, but not following gastric banding (156,157). Experimen-tal evidence suggests that these anorectic gut hormones may mediate the effects of RYGB on appe-tite and body weight (157,158). Post-prandial PYY and GLP-1 levels begin to rise as early as 2 days following gastric bypass in humans (158), and secretory products of enteroendocrine L-cells, including PYY and GLP-1 remain elevated two years after bypass surgery (159). Inhibiting gut hormone release with somatostatin analogue octreotide increases the food intake after gastric bypass surgery but not following gastric banding (158), further suggesting that these hormones play a critical role. In rodent models of bariatric surgery increases in circulating GLP-1 (160) and PYY and a reduction in ghrelin (49), have been implicated in mediating the beneficial effects of these surgeries. Determining the mechanisms behind the sustained reduction in appetite may identify pathways that can be targeted by anti-obesity agents. To this end there has been recent concerted effort to mimic the rise in gut hor-mones following gut bypass by either the development of peptide based analogues or by the design of small molecule drugs which target nutrient sensing receptors on the enteroendocrine L-cell.
Long acting versions of PYY and OXM are being actively pursued by the pharmaceutical industry, such as Pfizer’s OAP-189, we await the dissemination of data from ongoing trials. In addition Given that gut hormones are co-released one logical approach would be the development of combination therapies. Indeed data suggests that co-administration of gut hormones can have additive effects on food intake inhibition, for example PYY + GLP-1 (161) or PYY + OXM (162). Such combination ap-proaches may prove more effective than individual administration. Very recently the development of chimeric agonists has emerged as a novel form of combination therapy (91,92). GLP-1/glucagon co-agonists combine the appetite suppressive effects of GLP-1 and glucagon with the energy expendi-ture promoting effects of glucagon. Whilst at the same time GLP-1’s insulinotropic effects inhibit the detrimental hyperglycaemic effects of glucagon. Marcadia Ltd., now a subsidiary of Roche, first re-ported the beneficial effects of this approach and their compound is now undergoing clinical trials. In addition, Zealand Pharma is also developing a similar compound, ZP-2929, in partnership with Boe-hringer Mannheim. Time will tell if the promising pre-clinical data translates in to clinical benefit (163).
Considerable energy has also been directed toward the development of gut hormone secretagogues. The most well characterised class being agonists of GPR119. These compounds have been shown to release both GLP-1 and PYY (22). Their anti-diabetic effects are well defined; stimulation of GLP-1 and a direct insulinotropic action (22,164). It is less clear if these compounds will be effective as anti-obesity agents, but some agonists have been shown to significantly reduce food intake, for example PSN632408 (165). GPR119 is currently the only target for which synthetic modulators stimulate both incretin and insulin release. This highly beneficial profile has generated great industry interest with at least 9 companies actively working in this area. Initial clinical trials have been successful with respect to the anti-diabetic indication (166,167).
Conclusion
Obesity has emerged as a major global healthcare challenge. The significant mortality and morbidity associated with obesity has inspired a vast amount of research directed towards developing safe and efficacious weight-loss agents. The beneficial effects of centrally acting weight-loss agents have been negated by their potentially hazardous effects on mood, reward, dependence and autonomic tone. Gut hormones, as outlined in this article, play an important role in the homeostatic control of food in-take and offer an alternative to centrally acting drugs. We believe that in time these approaches will develop clinically useful compounds which will offer a real answer to the ever growing burden of obesity.


