Introduction
The manually intensive activities of venous blood collection represent a leading source of variability throughout the total testing process (1). Spurious hemolysis, in particular, is the main cause of unsuitable samples in most clinical laboratories, where the frequency of hemolyzed serum or plasma can be as high as 3% of all samples referred for routine and stat testing, and may even reach 8% of all samples collected in the emergency department (ED) (2). Spurious hemolysis, also known as “in vitro” hemolysis, is commonly referred to as breakdown of erythrocytes during blood collection, handling, transportation and storage (3). In routine laboratory practice, spurious hemolysis is typically classified according to concentration of cell-free hemoglobin that may be present in serum or plasma, so that it can be considered “mild” for hemoglobin concentration comprised between 0.5-1.0 g/L (pink to slightly red hue), “frank” for hemoglobin concentration between 1.0-3.0 g/L (red hue), and “gross” for hemoglobin concentration > 3.0 g/L (red to brown hue), respectively (4). In the presence of gross hemolysis, all test results are virtually affected, no corrective formulas seem effective to overcome the bias, and samples should hence be rejected (5).
Among the various mechanisms that may cause spurious erythrocyte injury, collection of venous blood through intravenous lines (i.e., catheters) is indeed prevailing in short stay units such as EDs or intensive care units (ICUs), where this practice represents a faster, easier and more convenient alternative than searching for another venipuncture site (6). The postulated mechanisms supporting a higher chance of hemolysis when blood is drawn from catheters include the presence of valves inside the collection system, the collapse of tube for high pressure aspiration, the placing of catheters in unusual sites (e.g., smaller distal veins) and use of large needles (e.g., greater than 20 gauge). Another potential mechanism is attributable to the higher shear stress caused by difference of pressure between veins and evacuated tube systems (6). Some potential solutions have already been proposed to overcome this last aspect, including the use of low volume vacuum tubes (7,8) or the manual aspiration of blood using syringe or closed collection systems such as S-Monovette® (Sarstedt AG & Co., Nümbrecht, Germany) (9,10), which may globally reduce the burden of spurious hemolysis.
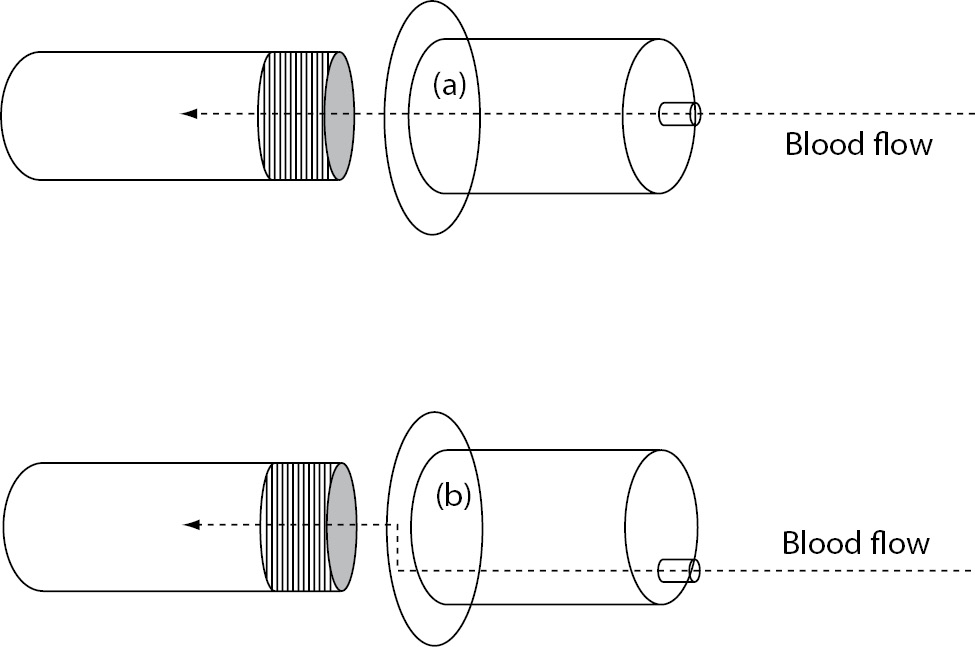
Another potential solution is represented by the use of specific collection systems that may be capable to attenuate the difference of pressure between vein and vacuum tubes. The Holdex® (Greiner Bio-One GmbH, Kremsmuenster, Austria) is a sterile, transparent disposable tube holder made from polypropylene, which is characterized by an eccentric luer on the top and a stainless steel needle inside the holder (Figure 1). Due to this specific structure, the blood flow through the system encounters two angles of 90°, which - according to the manufacturer’s declaration - would attenuate the delta of pressure existing between vein and vacuum tube when collecting blood from catheters, ultimately preventing erythrocyte injury, so that we planned a specific study to support this hypothesis.
Figure 1. Structure of (a) conventional evacuated tube holder (i.e., BD Vacutainer One Use Holder) and (b) Holdex.
Materials and methods
Study population
This randomized prospective investigation was settled in a large urban ED, averaging ~90,000 visits per year. The study population consisted in 60 consecutive patients admitted from 8:00 AM to 2:00 PM of a single working day, who required blood collection for diagnostics purposes, including assessment of potassium and lactate dehydrogenase (LD). Each patient provided an informed consent for being enrolled in this study, which was in accord with the ethical standards established by the institution in which the experiments were performed and the Helsinki Declaration of 1975.
Study design
According to our experimental design, blood was drawn by the same two nurses in duty at the ED through a 1.0 x 3.2 mm, 20-gauge catheter (Neo DELTA VEN, Viadana, MN, Italy; ref. n. 1331), placed in a vein of the upper limb.
In patients with odd order numbers (i.e., patients 1, 3, 5, etc), three consecutive 13 x 100 mm, 5.0 mL Vacutainer® SST II Plus serum tubes (Becton Dickinson Italia S.p.A., Milan, Italy) were collected. The first and the second tubes were drawn using a conventional Becton Dickinson (BD) tube holder (BD Vacutainer One Use Holder, Becton Dickinson), whereas the third tube was collected using Holdex® (Greiner Bio-One GmbH, Kremsmuenster, Austria). In the even group of patients (i.e., patients 2, 4, 6, etc.), the first and the second tubes were collected using Holdex, whereas the third tube was collected using BD Vacutainer One Use Holder. In the patients, the first serum tube was filled immediately after catheter insertion and then discarded, whereas the following tubes were transported to the core laboratory within 20 to 40 min after collection, where they were subjected to standard centrifugation according to manufacturers’ instructions (1300 x g for 10 min at room temperature). All blood tubes were collected and filled up to the nominal volume, and all phases of sample collection were accurately standardized.
Methods
Serum was tested for potassium (indirect ISE), LD (DGKC method) and hemolysis index (i.e., HI) on Beckman Coulter DxC, using proprietary reagents (Beckman Coulter Inc., Brea CA, USA). In this instrument, the HI is assessed by direct spectrophotometry, and a previous study has proven that this measure provides results of cell-free hemoglobin that are comparable with those of the reference cyanmethemoglobin assay (11). All tests were performed in duplicate, and results were finally averaged.
Statistical analysis
Normality distribution was verified by Kolmogorov-Smirnov test and concentrations were presented as mean and 95% confidence intervals (95% CI). The statistical analysis included Wilcoxon test for paired samples (for continuous variables), chi-squared test (for categorical variables) and analysis of bias by Bland & Altman plots. The statistical evaluation was performed with Analyse-it for Microsoft Excel version 2.24 (Analyse-it Software Ltd, Leeds, UK). Statistical significance was set at P < 0.05.
Results
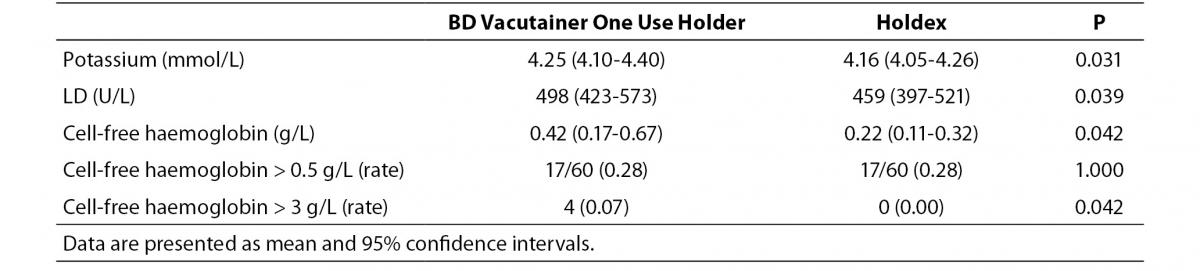
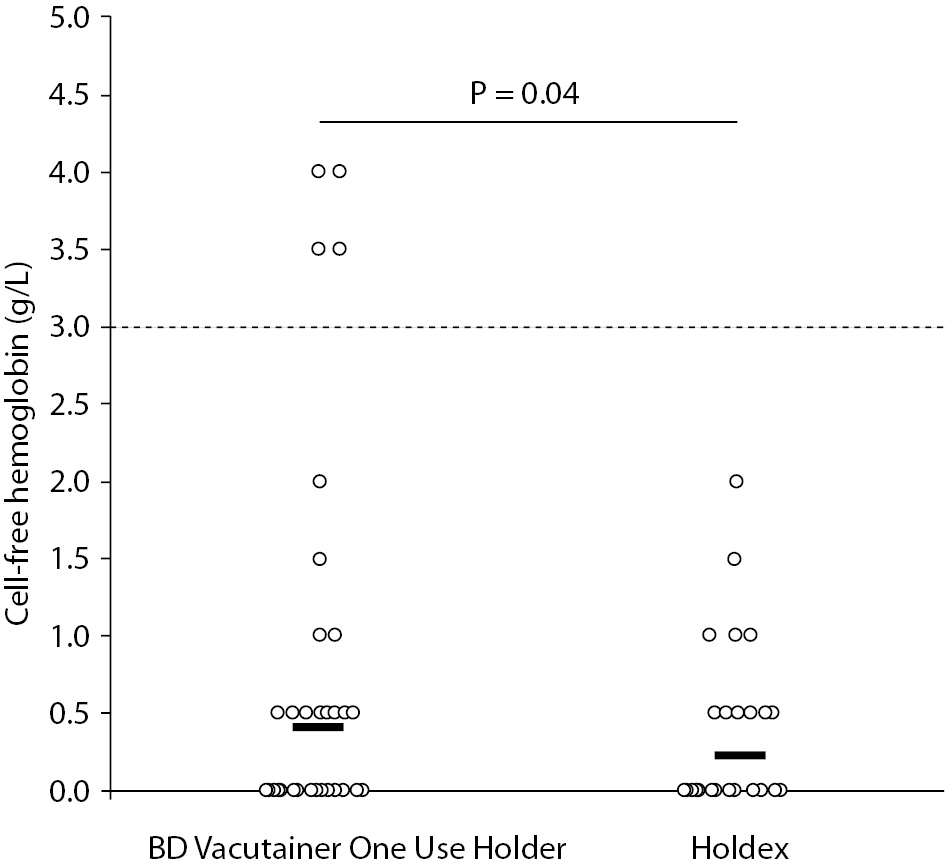
The main results of this study are synthesized in table 1. The final study population consisted in 60 ED patients (32 females and 28 males; median age 52 years, range 37-74 years). The sites of catheter insertion were the median cubital or basilic veins (22/60; 0.37), the median antebrachial vein (15/60; 0.25), cephalic vein (12/60; 0.20), basilic vein (5/60; 0.08) and veins of the metacarpal plexus (6/60; 0.10). In four patients the insertion was unsuccessful in the median cubital vein, and needed to be repeated on the opposite arm. Activity of LD and the concentrations of potassium and cell-free hemoglobin were found to be significantly higher in samples collected with the conventional BD Vacutainer One Use Holder than using Holdex (Table 1). The mean bias of cell-free hemoglobin in samples collected with Holdex was -0.4 g/L (95% CI, -0.9 to 0.1 g/L) as compared with BD Vacutainer One Use Holder. Although no statistically significant difference could be observed in the frequency of samples with concentration of cell-free hemoglobin > 0.5 g/L (i.e., 17/60 vs. 17/60; Pearson’s X2 statistic: 0.00; P = 1.000), the frequency of samples with gross hemolysis (i.e., > 3.0 g/L, found in 2 samples collected from veins of metacarpal plexus, 1 from median basilic and 1 from cephalic veins) was significantly higher in samples collected with BD Vacutainer One Use Holder than with Holdex (i.e., 4/60 vs. 0/60; Pearson’s X2 statistic: 4.14; P = 0.042) (Figure 2).
Table 1. Concentration of potassium and cell-free haemoglobin and lactate dehydrogenase (LD) activity in serum samples drawn from intravenous line using a conventional tube holder (BD Vacutainer One Use Holder) and Holdex.
Figure 2. Distribution of cell-free hemoglobin concentration in serum samples drawn from intravenous line using a conventional tube holder (BD Vacutainer One Use Holder) and Holdex. The horizontal dotted line defines the threshold of gross hemolysis.
Discussion
Spurious hemolysis is a major issue in laboratory diagnostics, due to its relatively high frequency and the economical, organizational and clinical issues that may be associated with receipt of hemolyzed blood for routine or urgent testing (3,4). The presence of a large amount of cell-free hemoglobin in serum or plasma (i.e., exceeding 3.0 g/L) may in fact cause suppression of virtually all tests, since the bias may cause an unavoidable degree of biological and/or analytical interference (5). The identification of potential solutions that may be effective to overcome this frequent problem is hence advisable.
It has been clearly established that blood collection through intravenous lines is an important cause of erythrocyte injury, which has been at least partially attributed to remarkable difference of pressure existing between vein and vacuum tubes (6). The development of tube holders specifically suited for collecting blood from intravenous lines would hence represent a breakthrough for decreasing the risk of obtaining unsuitable samples and consequently lowering healthcare costs and patient discomfort. The difference between the Holdex tube holder and other standard devices is represented by the eccentric luer needle adapter, which allows a flat venipuncture angle (Figure 1). One advantage of this system is represented by the fact that the blood can be seen clearly flowing through the translucent adapter when venipuncture has been successfully carried out. Another claimed benefit is the lower risk of producing spurious hemolysis when collecting blood from catheters or butterfly needles. In fact, while in a standard holder the blood follows a direct linear flow from the venipuncture site into blood collection tubes, the blood flow in Holdex is interrupted by the presence of two angles of 90°, which finally attenuate the gap of pressure existing between veins and vacuum tubes.
In a previous study, Almagor and Lavid-Levy assessed within-subject variations in the results of some blood analyses in blood collected by straight needles using Greiner or the BD systems, and found that plasma hemoglobin was higher using the former system (12). Although the authors theorized that the variation in linear flow of blood may generate a mechanical strain on blood cells, thus affecting membrane integrity and causing efflux of intracellular constituents into the surrounding serum, the difference was globally modest (i.e., 0.25 vs. 0.16 g/L; P < 0.025) and still lower than 0.5 g/L, that is the conventional threshold of “mild” hemolysis. In a following cross-sectional study, Romero Ruiz et al. compared blood collection by standard venipuncture using Holdex, BD system and syringe, and showed that the rate of hemolysis did not differ among the three collection systems (i.e., 16% with Holdex, 15% with BD and 23% with syringe) (13). In both studies, however, no data were provided about the use of Holdex for collecting blood from intravenous lines.
The results of this study, which is the first that has assessed the impact of Holdex on collecting blood from catheters, show that although the typical structure of the system may be a (relative) drawback when collecting blood with straight needles (12), it may turn to be a tangible advantage when blood is drawn from intravenous lines. It is noteworthy, however, that the distribution of concentrations was largely overlapping and that the P values were also modest, so that the global effect must be weighed against these data. Although the overall rate of visually hemolyzed specimens (i.e., cell-free hemoglobin > 0.5 g/L) was identical (i.e., 0.28), the frequency of those with gross hemolysis (i.e., cell-free hemoglobin > 3.0 g/L) was substantially higher using a conventional tube holder than with Holdex. It is also noteworthy that the concentration of cell-free hemoglobin, as well as that of two indices of hemolysis such as potassium and LD activity, was also higher in samples drawn with a conventional tube holder, thus implicitly confirming that the erythrocyte injury may be attenuated with this novel device. We thereby conclude that use of Holdex for collecting blood from intravenous lines may be mildly effective to reduce the risk of obtaining grossly hemolyzed specimens.
Acknowledgements
The authors acknowledge the nurses Maria Pia Carpi, Caterina Colombo and Davide Caputo for the skill assistance during blood collection in the emergency department.