Introduction
Ethanol is the most frequent cause of substance-related traffic accidents, and driving under the influence of alcohol is a severe social problem (1). Increased blood ethanol concentrations in drivers are directly related to increased risk of traffic accidents. Moreover, in traffic accidents, measurement of blood ethanol concentrations can be a deciding factor when determining who was at fault for the accident. Thus, measurement of blood ethanol concentrations needs to be accurate and reliable (2).
Despite the widespread nature of driving under the influence, there are still problems sample collection, transportation, and storage that may interfere with the analysis of ethanol. The preanalytical phase (i.e., collection, transport, and storage) has a substantial role in the quality and reliability of analytical ethanol results, which depend on the quality and acceptability of specimens. Additionally, these pre-analytical steps (proper sampling, handling, and storage of blood ethanol samples) are critical in forensic cases (3,4). For blood ethanol testing, current standards are described by the Clinical and Laboratory Standards Institute (CLSI) (5). Measurement of ethanol can be performed on various body fluids in living, and deceased subjects. While antecubital venous blood samples are the most suitable sample type for living subjects, femoral venous blood samples are more suitable for deceased subjects during autopsy (6). Moreover, it is essential to properly cleanse the venipuncture site using a disinfectant that does not contain alcohol or other volatile organic substances. The most frequently used disinfectants for this purpose are aqueous benzalkonium chloride or povidone-iodine (3,5). Dubowski found that blood specimens can be significantly contaminated if alcohol-containing materials are used to cover the venipuncture site (7). The most frequent samples used by clinical biochemistry laboratories to analyze ethanol concentrations are the whole blood, plasma, or serum (8).
Although processes for medical examinations, treatments, and reporting of forensic cases are defined by legal arrangements with regard to healthcare aspects, laboratory services and the framework of medicolegal responsibilities have not been clearly defined in current legal arrangements, both in our country and in many other countries worldwide. The processes for sampling, accepting, handling, and storing of specimens belonging to forensic cases have not been standardized, and unexpected and confusing results may lead to erroneous interpretations. Therefore, each laboratory should establish its own definitions for reliable processes. Additionally, current standards and laws have not provided clear instructions for the storage of “replicate samples”. The stability of blood ethanol during storage is an important problem if replicate samples need to be re-analyzed after storage, which may be required upon a request by the judicial authority to confirm initial results. To date, several studies have investigated the stability of blood ethanol at different storage temperatures and for different storage durations (9-12). However, no clear standards for the storage and re-analysis of blood ethanol have been established.
In the present study, we aimed to analyze changes in ethanol concentrations in blood samples after storage for different durations, and, based on the results, we attempted to establish a standard protocol for storage of blood samples for subsequent analysis of blood ethanol levels.
Materials and methods
Study design
This retrospective study was carried out at Evliya Celebi Research and Education Hospital of Dumlupinar University, Turkey from November 2013 to April 2014. The study was carried out in accordance with Declaration of Helsinki. Samples with ethanol concentrations greater than 2.17 mmol/L (> 0.1 g/L) were included in the study (N = 80). This value (0.1 g/L or 2.17 mmol/L) is lower detection limit for the reagents used in present study. Blood samples that were hemolysed, clotted, collected after too long of a wait, under- or overfilled, or collected in improper tubes were rejected, and appropriate samples were requested. After blood samples were collected from intoxicated drivers, samples were transported to the laboratory. Blood samples were then aliquoted; one of the sample aliquots was immediately analyzed, while the other sample aliquots were stored at -20 °C until re-analysis. Stored plasma samples were grouped according to storage period as follows:
- group I (G I) samples were stored at -20 °C for up to 5 months (N = 20);
- group II (G II) samples were stored at -20 °C for up to 4 months (N = 20);
- group III (G III) samples were stored at -20 °C for up to 3 months (N = 20), and
- group IV (G IV) samples were stored at -20 °C for up to 2 months until re-analysis (N = 20).
These groups were composed of different plasma samples to prevent ethanol losses occurring after repeated opening of tube caps.
Sample collection and measurement of plasma ethanol concentrations
Whole blood samples were drawn into 4-mL evacuated tubes with gray rubber stoppers containing sodium fluoride plus K3EDTA as additives (Vacuette, Greiner Bio-One, Kremsmunster, Austria) to prevent coagulation and fermentation according to CLSI standards (5). First, the whole blood samples were inverted gently at least 8–10 times for mixing with additives, then samples were immediately centrifuged at 3000 × g for 15 min. Plasma samples were aliquoted into two separate polystyrene tubes. One of the plasma aliquots was immediately analyzed, and the other plasma aliquots were stoppered air tight and stored at -20 °C until re-analysis. The frozen samples were re-analyzed synchronically over the course of 1 day. Before re-analysis, frozen samples were thawed to room temperature. The plasma ethanol concentrations were measured on a Roche Cobas C 501 analyzer (Roche Diagnostics GmbH, Mannheim, Germany) using original Roche commercial reagents (Roche Diagnostics GmbH) according to the alcohol dehydrogenase method (13). Tests were performed in duplicate and internal quality control samples were included in each assay run. Intra-assay coefficients of variation (CVs) were 8.27% (mean ± SD: 11.28 ± 0.93) and 2.36% (32.40 ± 0.76), with target values of 10.9 mmol/L and 31.6 mmol/L.
According to the manufacturer’s instructions for the reagent and analyzer, ethanol concentrations remains stable if the tubes are kept at room temperature (15-25 °C) for 2 days, at 2-8 °C for 2 weeks, or at -25 to -15 °C for 4 weeks (14).
Statistical analysis
All data sets were tested for normality using Kolmogorov-Smirnov tests. Initial and poststorage ethanol concentration values were normally distributed in each group; however, because the sample size was less than 30 (N = 20), the difference between the initial and poststorage plasma ethanol concentrations for each group was analyzed using two-tailed Wilcoxon matched-pairs tests. Data were presented as median and interquartile range (IQR). The deviation (%) from the initial concentration was calculated using Microsoft Excel (Microsoft Office 2010, Microsoft, Redmond, WA, USA). The deviation was calculated according to the following equation:
formula bias (%) = [(meantarget – meanmeasured)/meantarget] × 100%.
The calculated deviations were evaluated with the allowable total error (TEa) according to the Clinical Laboratory Improvement Amendments (CLIA’88) Proficiency Testing Limits, U.S. Federal Register (TEa ± 25%) (15). The relationships between the initial concentrations and deviations from the initial concentrations (%) were analyzed by Spearman’s correlation coefficient. For all statistical tests, differences with Pvalues of less than 0.05 were considered statistically significant. All analyses were performed using SPSS software (version 13.0 for Windows; SPSS, Inc., Chicago, IL, USA).
Results
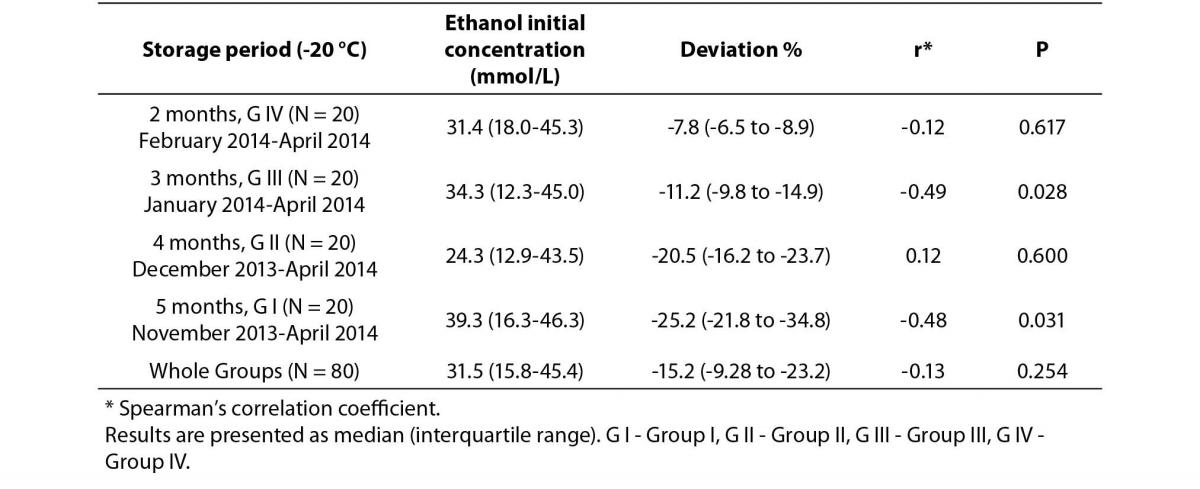
Decreases in plasma ethanol concentrations were observed in all four groups of samples with different storage conditions. The differences between the initial ethanol concentrations and post-storage concentrations are shown in Table 1. A statistically significant difference was observed for the overall sample set (P < 0.001). The relationships between the initial ethanol concentrations and the deviations from initial concentrations (%) are shown in Table 2. Statistically significant negative correlations were observed only in G I and G III (r = -0.48, P = 0.031 and r = -0.49, P = 0.028, respectively).
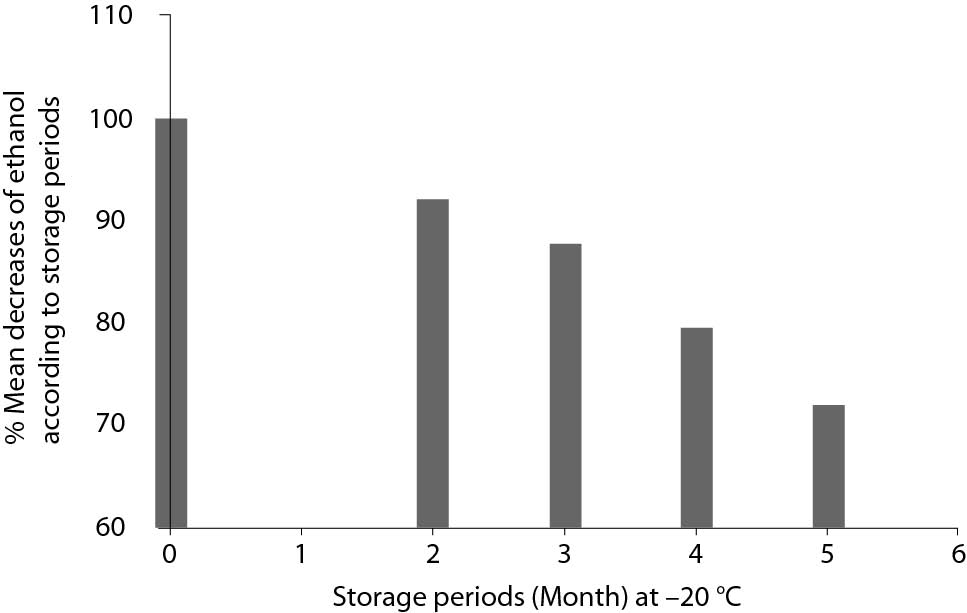
Mean decreases (%) in plasma ethanol concentrations from initial concentrations according to storage duration and comparisons with TEas according to CLIA’88 (± 25%) are shown in Figure 1. Deviations from the initial concentrations that exceed the TEa were observed in G I (in 11 of 20 tubes) and G II (in 4 of 20 tubes); these results were considered as analytically significant. The deviations were within the acceptable ranges in G III and G IV; therefore, these results were considered not analytically significant. Additionally, the mean decreases in ethanol concentrations were directly proportional to the storage period. Mean decreases (%) in ethanol concentrations according to storage periods are shown in Figure 2.
Discussion
The stability of blood ethanol over time is an important problem if samples are required to be re-analyzed after storage, particularly after an extended period (16). Therefore, in this study, we investigated the stability of blood ethanol concentrations over time.
Table 1. Comparisons of initial and post-storage plasma ethanol concentrations.
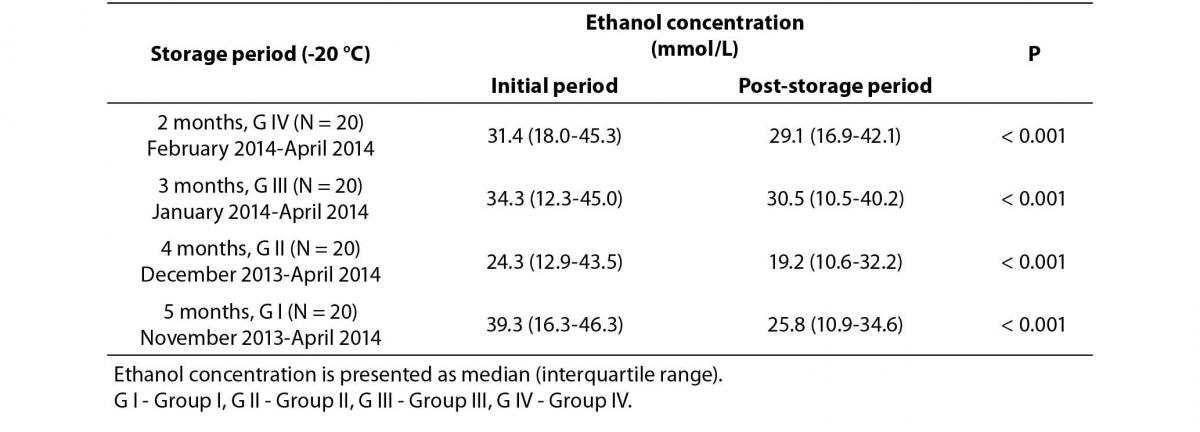
Table 2. Correlation analysis between initial plasma ethanol concentrations and deviations from initial concentrations
Importantly, the most significant ethanol losses occurred in G I and somewhat in G II, and the mean deviation (%) values were analytically significant in these two groups. Additionally, while the instructions for the manufacturer of the reagents states that ethanol should be stable for up to 4 weeks when stored at -15 to-25 °C (14), we found that samples were sufficiently stable at -20 °C for up to 3–4 months. Decreases in ethanol concentrations were directly proportional to the duration of the storage period. Finally, when we analyzed the correlation between the percent decreases from initial concentrations and the later measurements of ethanol concentrations, we found statistically significant negative correlations in G I and G III (P = 0.031, r = -0.48; and P = 0.028, r = 0.49, respectively).
Ethanol concentrations may increase or decrease as a consequence of ethanol loss or endogenous ethanol formation during the storage of samples. Ethanol losses are largely attributed to evaporation, chemical oxidation, and microbial consumption, whereas increases are largely due to microbial conversion of substrates, such as glucose to ethanol through fermentation (16,17). Increases in ethanol concentrations due to synthesis of ethanol by microorganisms can be prevented by using collection tubes that contain preservatives, such as sodium fluoride (NaF). Substrates such as glucose and viable microorganisms lead to production of ethanol through fermentation if the sample is placed in a collection tube without preservatives, such as enzyme inhibitors (18). In our study, no ethanol was generated in any of the samples over the 5-month storage period.
Figure 1. Mean decreases (%) in plasma ethanol concentrations from initial concentrations according to periods of storage, and comparisons with allowable total error (TEa) according to CLIA’88 (± 25%).
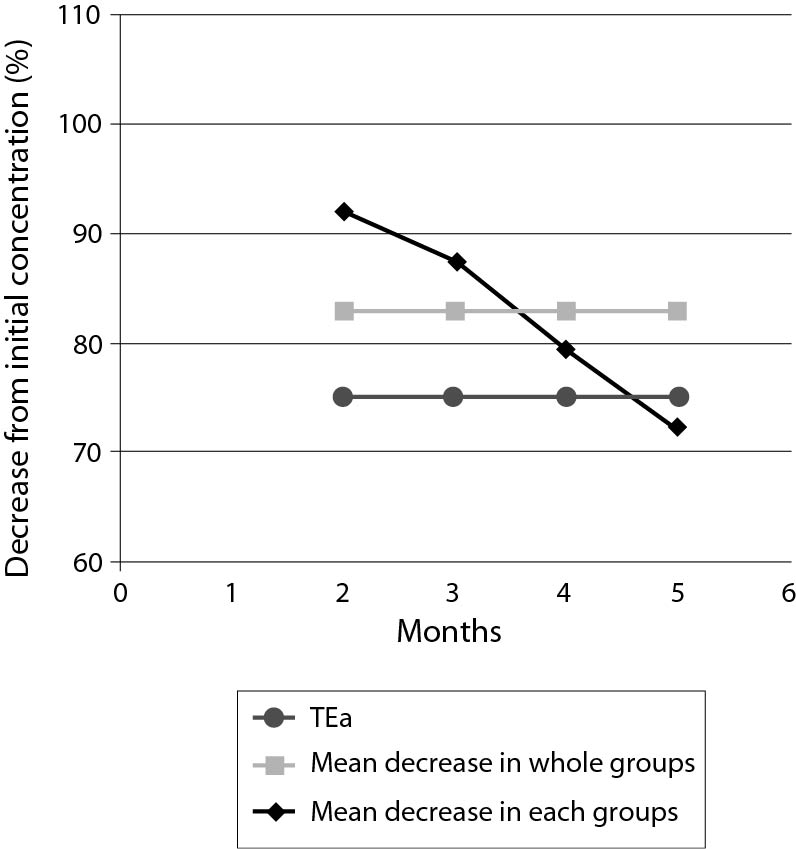
Figure 2. Mean decreases (%) in plasma ethanol concentrations according to storage periods.
Previously published studies have revealed that the stability of ethanol in the blood depends on some specific factors, such as storage period, concentrations and types of additives, temperature during the storage period, air quantity above the sample, and tightness of stoppers for the containers used. However, the blood ethanol stabilities reported in these studies are not consistent (10,19-23). Brown and colleagues found that ethanol losses occurred via microorganisms in the absence of preservatives, which could be prevented by 0.5% NaF. Additionally, they found that ethanol oxidation was dependent on storage temperature and that diffusion occurred from 5.6% of the polypropylene container; these data indicate that the most important factors affecting blood ethanol stability are temperature, NaF concentration, and duration of storage (10). Winek and Paul found that samples did not show significant gains or losses in ethanol concentrations with changes in storage duration (up to 14 days), temperature, and NaF; they concluded that blood ethanol analysis could be delayed for as long as 14 days without significant changes in ethanol, regardless of whether the samples were refrigerated or whether preservatives were added (19). Additionally, Penetar demonstrated that serum and plasma ethanol concentrations were higher (11%) than whole blood samples (20). Also, neither different processing conditions nor different storage conditions significantly affected ethanol concentrations. Blood samples containing ethanol at levels ranging from 60 to 90 mg/dL were not significantly affected by the type of collection tube used or the storage conditions during a 10-day period (20). In an analysis of storage for up to 6 months, Meyer and coworkers indicated that freezing is a suitable method for storage of blood samples containing ethanol for forensic purposes over reasonable durations (21). Additionally, they found that ethanol formation did not occur in blood samples containing preservatives (21). Jones examined changes in blood ethanol concentrations during storage in evacuated tubes containing NaF and potassium oxalate at 4 °C for up to 12 months. Ethanol concentrations decreased significantly by 2.6%, 1.9%, and 1.8%, respectively, at initial concentrations of 0.5, 1.5, and 2.5 mg/g (22). Furthermore, the mean decreases in blood ethanol concentrations between initial measurement and re-analysis were significant (P < 0.001), and the loss of ethanol was positively correlated with the duration of storage (22). In a separate study, significant decreases in ethanol levels occurred in samples stored at room temperature for up to 7 or 14 days (7.9% and 22.4% decreases, respectively). Additionally, significant decreases also occurred in samples stored at 4 °C for up to 1–3 months and for more than 3 months (4.2% and 7.9% decrease, respectively). However, while no statistically significant decrease was found in samples stored at -20 °C for up to 1–3 months (only a 4% decrease), statistically significant decreases were observed in samples stored at -20 °C for more than 6 months (a 15% decrease) (23).
Thus, the results of previously published studies are not consistent regarding suitable conditions for storage of blood ethanol samples. The results of our study were consistent with those of Brown, Jones, and Mandic-Radic (10,22,23). According to our results, the decreases in ethanol concentrations were directly proportional to the duration of storage, similar to the results of the studies by Jones and Mandic-Radic (22,23). Moreover, in our study, samples with a low ethanol concentration showed a somewhat greater decrease from the initial concentration, in contrast to the results of Hayden and colleagues who found that greater decreases occurred in samples with higher ethanol levels (12). Our results were also similar to those of Saracevic and coworkers, who found that ethanol stability showed poor or no concentration dependency (24). In our study, although the blood samples were collected in tubes containing preservative (NaF) and stored at -20 °C, ethanol losses occurred in all groups. These ethanol losses may be due to escape of ethanol vapour from around the stopper of tubes and chemical oxidation of ethanol resulting from the presence of air above the sample. Even though the recommended proportion of air in sample tubes is 0%, this is not always possible. According to the study by Saracevic et al., while statistically significant ethanol decreases were found between the initial concentrations and re-analyzed concentrations in unstoppered tubes, no statistically significant differences were observed in closed tubes (24). Additionally, ethanol losses in our study may also occur due to the diffusion of ethanol from polypropylene tubes, as reported in the study by Brown (10).
Overall, the differences observed between our study and previously published studies are likely caused by different methods used to ethanol analysis, different numbers of samples, and differences in the environmental conditions of each laboratory. Therefore, each laboratory should establish its own work-flow rules and criterion for reliable ethanol results in forensic cases.
Conclusion
One of the most important factors affecting results of ethanol analysis is the storage of samples under suitable conditions of duration and temperature. Current standards and legislation do not address how blood samples should be stored as replicate samples after the first analysis. According to the results of this study, plasma ethanol samples can be stored at -20 °C for up to 3–4 months until re-analysis, providing that samples are kept in suitable sample tubes, which are tightly closed and nearly completely filled to minimize the amount of air above the samples. These results should provide the basis for the development of more appropriate standard practices for forensic science.


