Introduction
Chronic kidney disease (CKD) is a general term for heterogeneous disorders affecting kidney structure and function with variable clinical presentations. The Kidney Disease: Improving Global Outcomes (KDIGO) CKD Working Group released new updated KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease which pointed on the relevance of laboratory medicine in diagnosis of CKD (1). Early identification and management of CKD is highly cost-effective and can reduce the risk of kidney failure progression and cardiovascular disease by up to 50% and increasing the recognition of kidney damage is a key part of improving health outcomes in the community (2). The prerequisite for screening policies for chronic kidney disease is standardization of measurement of serum creatinine and automatic reporting of estimated glomerular filtration rate (eGFR) consequently as a marker of kidney function, and uniform assessment of albuminuria (or proteinuria) type of sample used and reporting units as a marker of kidney damage, nationwide (3). In 2014 Joint Croatian Working Group (JCWG) for laboratory diagnostics of CKD on the behalf of Croatian society of medical biochemistry and laboratory medicine (CSMBLM) and Croatian chamber of medical biochemists (CCMB) initiated proceedings to provide easily applicable national recommendations in this area of laboratory medicine. The initial step was to assess the current practice among Croatian laboratories in CKD evaluation, in order to set the background for developing national recommendations. The aim of this study was to assess the degree of adherence of medical laboratories in Croatia with KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease.
Materials and methods
Questionnaire
An e-mail invitation to participate in the on-line survey was sent to all Croatian medical biochemistry laboratories (N = 196). The e-mail was addressed to laboratory managers in order to avoid duplicate answers from one laboratory. The on-line survey was performed using the SurveyMonkey tool (Palo Alto, CA, USA), and it was held from April 3rd until May 20th 2014, with two reminders in that period of time. The survey was anonymous.
Questionnaire was designed in a form of questions and statements, with possible multiple answers and comprised 24 questions (Table 1). The intention was to provide data about current practice in Croatian medical biochemistry laboratories regarding laboratory tests for initial screening of CKD: creatinine, eGFR and albuminuria or proteinuria. The questionnaire contained questions about all three phases of total testing process. Questions regarding preanalytical phase included preferred types of samples used for measuring urine albumin or protein. Analytical phase was covered with questions about methods, reagent manufacturer and instruments for measuring creatinine and urine albumin and protein, while postanalytical phase questions included specifying reference intervals (or cut-offs) and units of measurement and reporting for: serum creatinine, urine albumin and urine protein. Additional questions about equations for calculating eGFR, as well as reporting eGFR were included in the survey.
Table 1. Questionnaire.
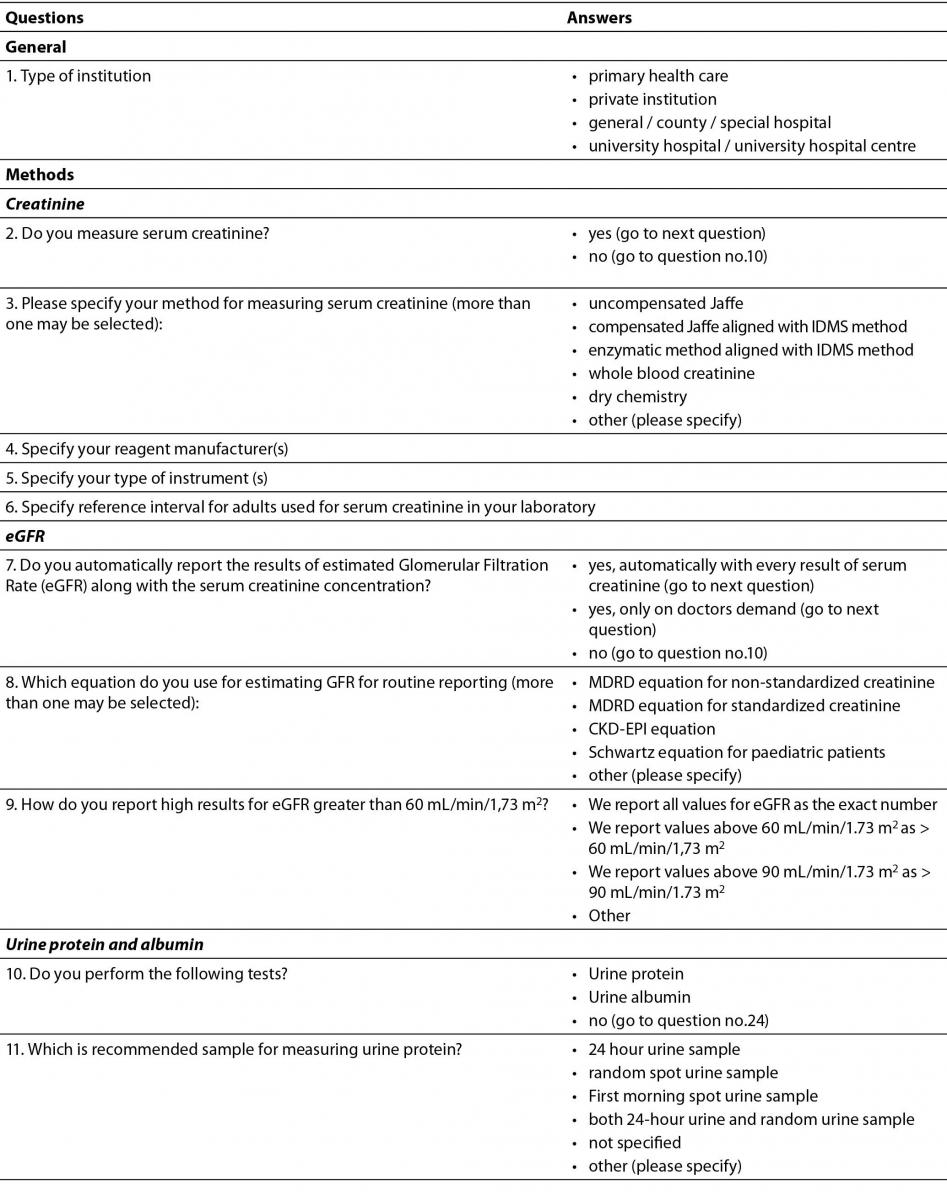
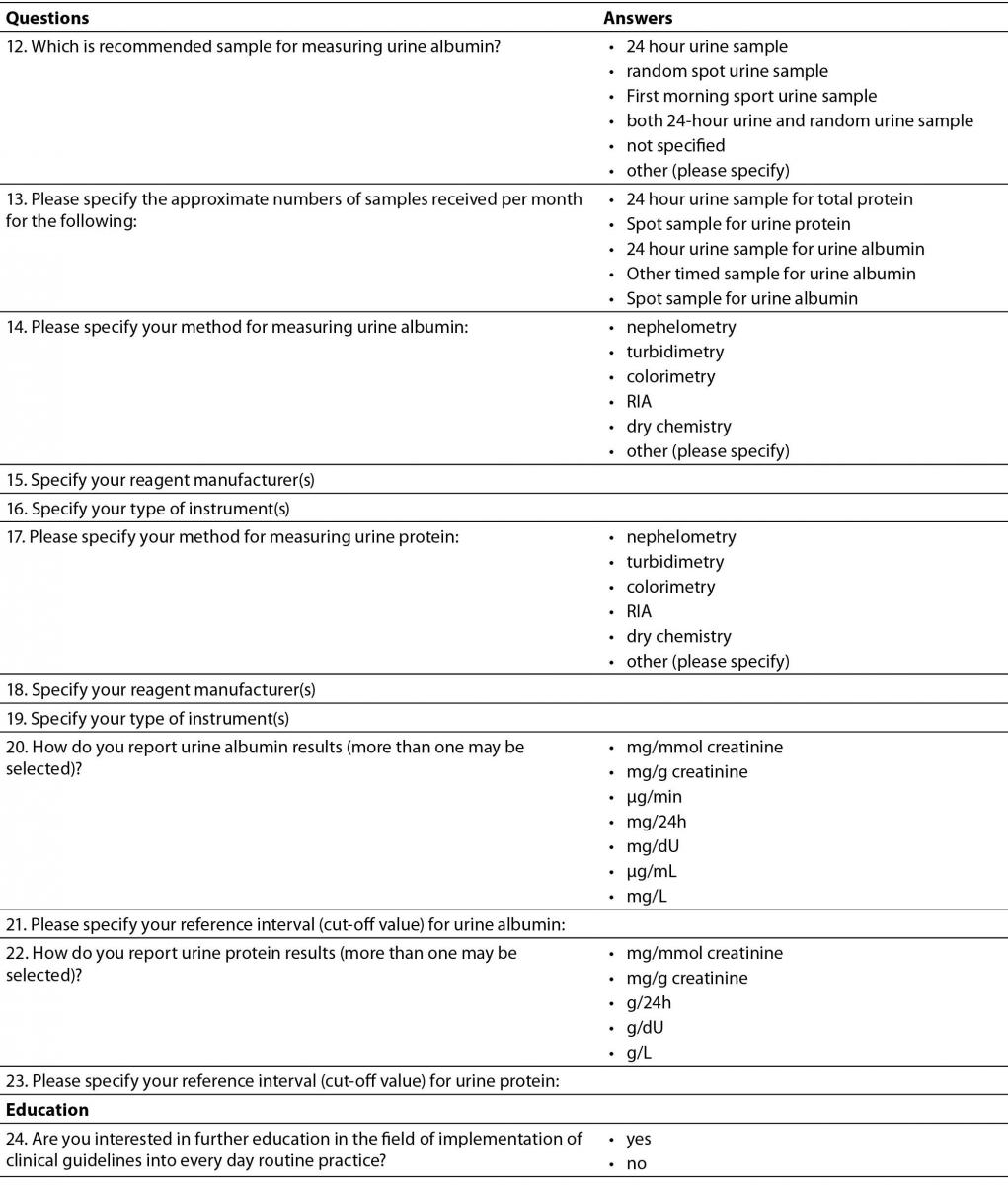
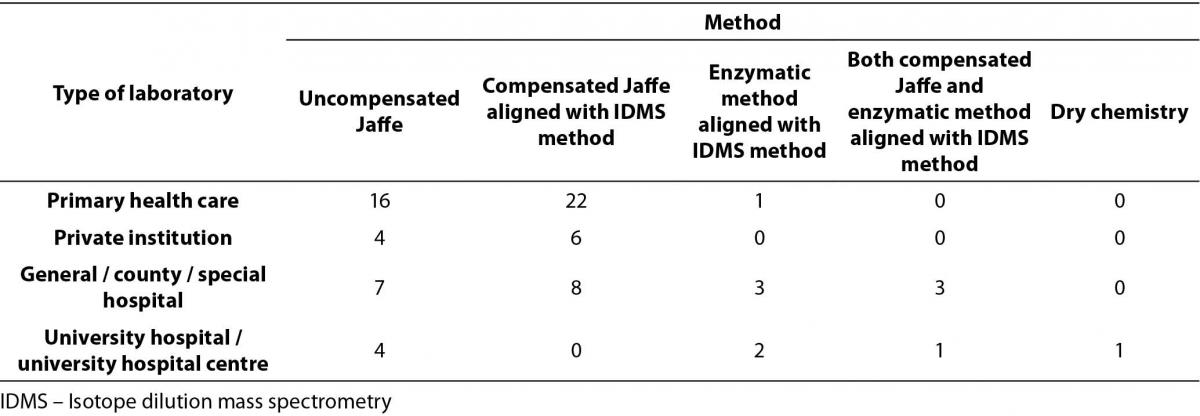
Table 2. Distribution of methods for serum creatinine measurement by type of medical biochemistry laboratory in Croatia (N = 78).
Statistical analysis
Data were archived in Microsoft Excell 2010 program (Microsoft. Microsoft Excel. Redmond, Washington: Microsoft, 2010. Computer Software). Results were provided by counting and presented as absolute numbers and percentages (or ratios). Distribution of answers subdivided into groups according to type of institution was compared with Chi-square test. Statistical analysis was performed using MedCalc 9.4.2.0. statistical software (MedCalc Software, Mariakerke, Belgium). P < 0.05 was defined as the threshold of significance.
Results
Total of 80/196 (40.8%) laboratories answered the questionnaire, out of which almost half were from primary health-care institutions (39/80). 22 laboratories from general/county/special types of hospitals answered the survey, 10 answers were from private laboratories (integral parts of private policlinics) and there were nine responders from clinical hospitals and clinical hospital centres.
Creatinine
Only 2/80 laboratories do not measure serum creatinine. The most prevalent method used for serum creatinine measurement was compensated Jaffé method traceable to reference Isotope Dilution Mass Spectrometry (IDMS) method and The National Institute of Standards and Technology Standard Reference Material (NIST SRM) 967 for creatinine in serum (40/78). Distribution of methods used for serum creatinine measurements, subdivided by types of institutions, is shown in Table 2. Comparison of methods across type of institutions was performed by Chi-square test. Considering the fact that there were low frequencies in some fields, methods for creatinine measurement were divided in two categories: non-standardized uncompensated Jaffé method and standardized methods (compensated Jaffé and enzymatic methods). This comprised our distribution table into 2 X 4 table. There was no statistically significant difference in distribution of methods by type of institution, P = 0.564. However, it is worth to emphasize that only one laboratory among primary health care laboratories, and none of the private laboratories, determine serum creatinine using enzymatic method.
The majority of laboratories (73/78) are using reference intervals according to the recommendations from the CCMB (4). However, a large proportion (16/78, 0.20) of laboratories are using incorrect reference intervals according to the stated method used for serum creatinine measurement. Five laboratories measure creatinine with uncompensated Jaffé method, but their reference intervals are for standardized creatinine. Eleven laboratories determine creatinine with standardized method (compensated Jaffé or enzymatic), but they are using reference intervals for non-standardized creatinine.
The most prevalent manufacturers of reagents used for serum creatinine measurement are Beckman Coulter (Beckman Coulter, Inc., Pasadena, California, USA) (34/78) and Roche (F.Hoffmann-La Roche, Basel, Switzerland) (28/78). Other reagents used were from the listed manufacturers: Siemens (Siemens AG, Berlin, Germany) (6/78), Herbos (Herbos d.d., Sisak, Croatia) (3/78), Diasys (DiaSys Diagnostic Systems GmbH, Holzheim, Germany) (3/78), Abbott (Abbott Laboratories, IL, USA) (2/78), Human (Human, Wiesbaden, Germany) (1/78) and Ortho Clinical Diagnostics (Ortho Clinical Diagnostics Inc., USA) (1/78). Only 10 out of 78 laboratories determine serum creatinine concentration using enzymatic method traceable to IDMS reference method. Six of them are using reagents from Beckman Coulter, and four of them Roche reagents.
eGFR
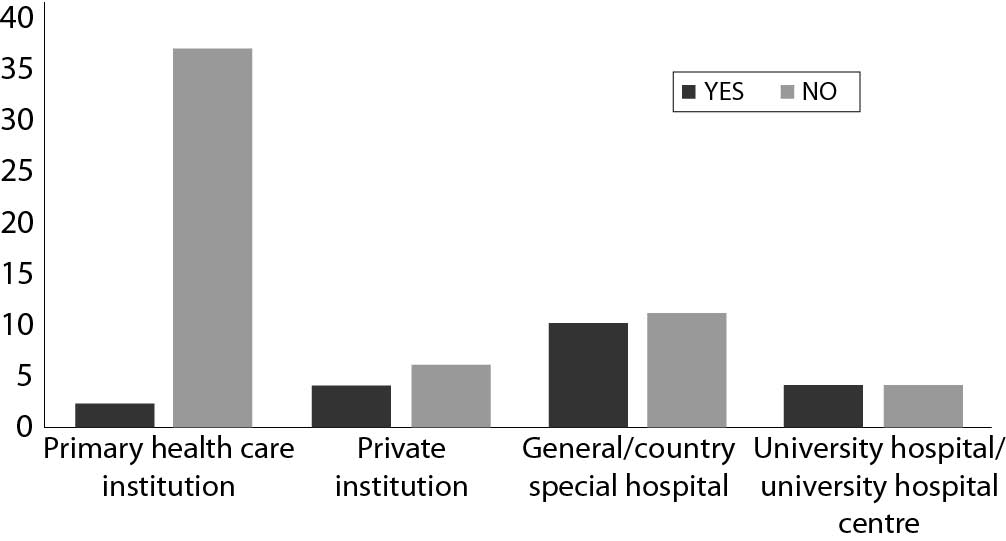
The majority of laboratories generally do not report results for eGFR (58/78). There was a statistically significant difference in distribution of laboratories that report eGFR by type of institution (P < 0.001), with the lowest number of laboratories from primary health care institutions (Figure 1.).
Figure 1. Comparison of number of laboratories that report eGFR values by type of institution in Croatia, N = 78.
Description of equations used for calculating eGFR by type of medical biochemistry laboratory, method for serum creatinine measurement, reporting units and additional comments are shown in Table 3.
Table 3. Description of used equations for calculating eGFR by type of medical biochemistry laboratory, method for serum creatinine measurement, reporting units and additional comments.*
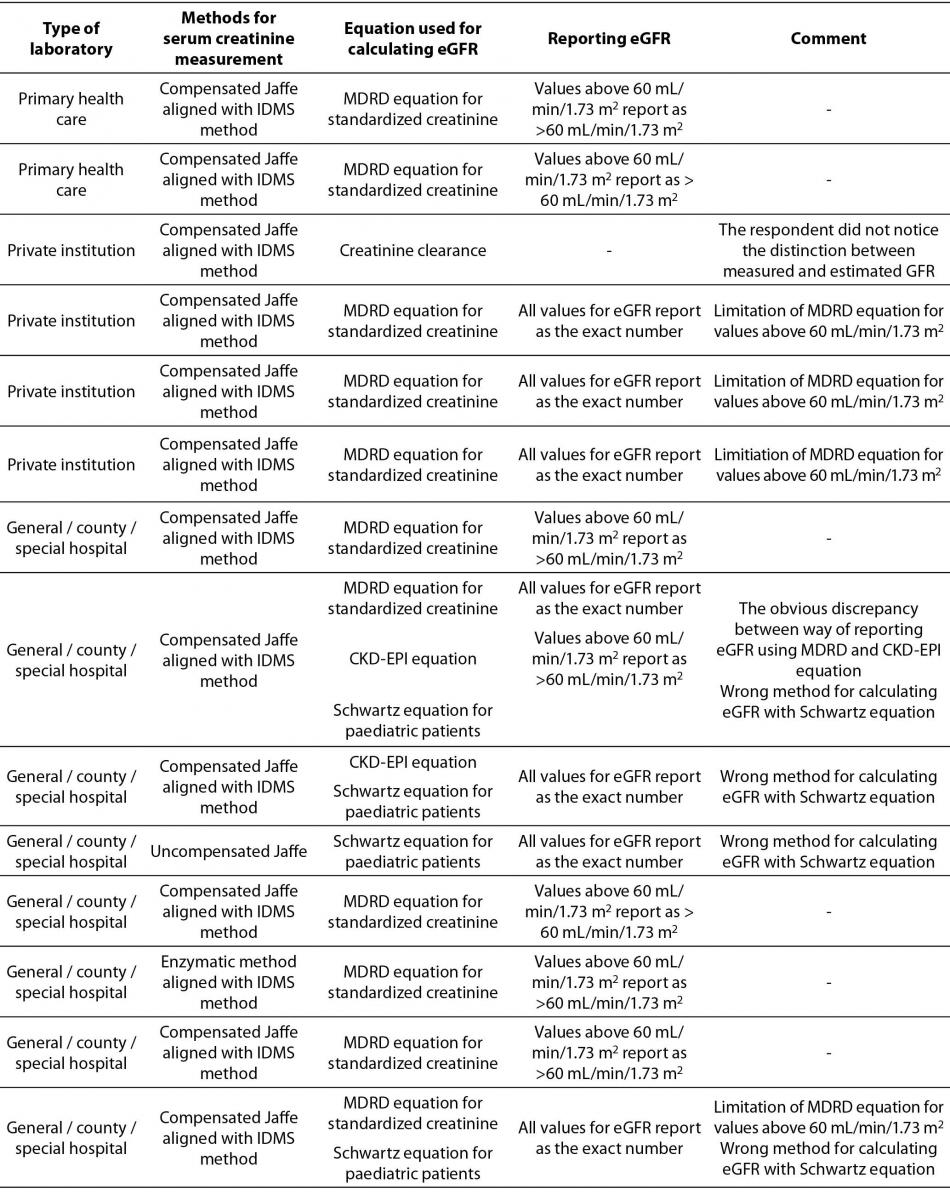
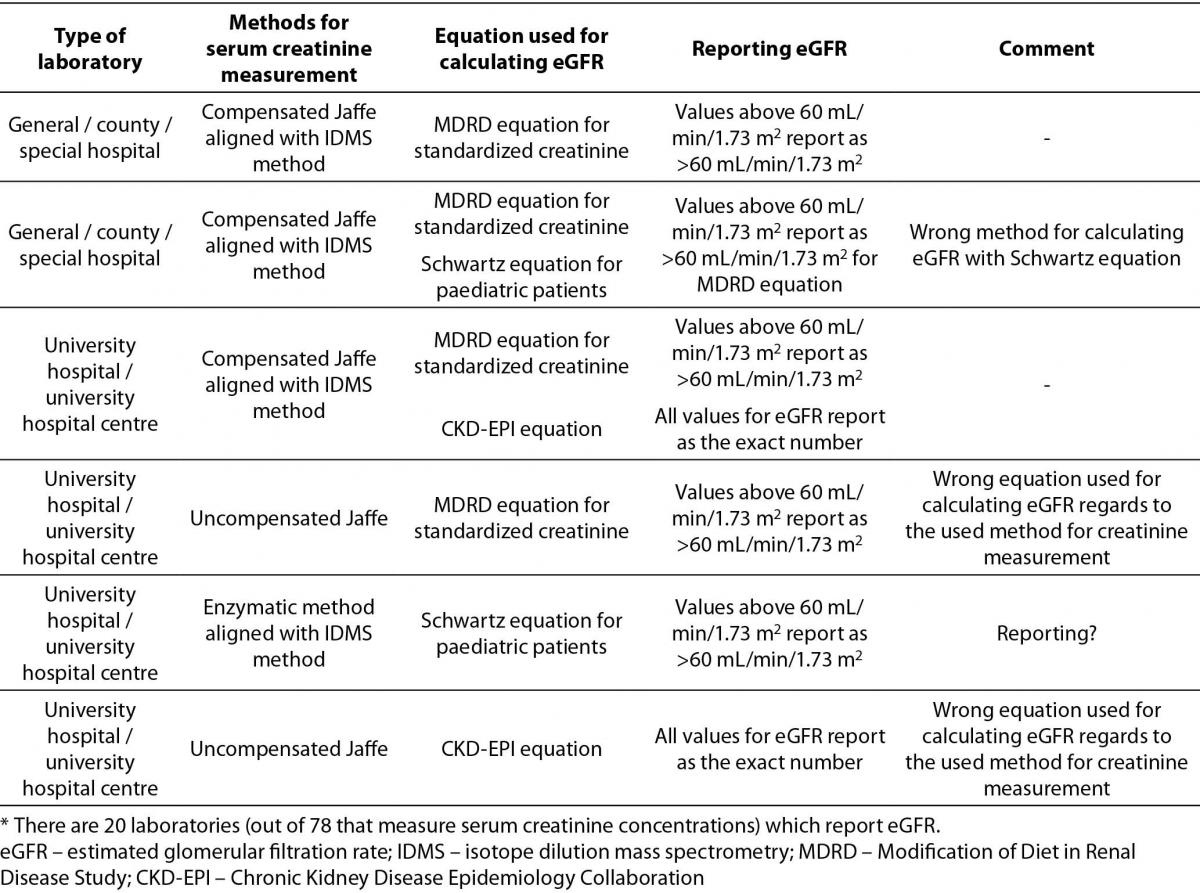
The most prevalent equation for calculating eGFR is Modification of Diet in Renal Disease Study (MDRD) equation for standardized creatinine (15/20). 5 laboratories are using more than one equation for calculating eGFR. 4 of them are using Schwartz equation for paediatric patients in combination with MDRD or Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation for adults. Only one laboratory combines MDRD equation for standardized creatinine with CKD-EPI equation for adult population. In the Table 3, it was clearly shown that there were some answers indicating using the MDRD equation for standardized creatinine with the results of serum creatinine measured with non-standardized uncompensated Jaffé method (2/20), and reporting of results for eGFR calculated with MDRD equation as an exact number regardless of eGFR value (4/20).
Albuminuria/proteinuria
Majority of laboratories that participated in the survey do not measure urine albumin or protein (58/80). Out of those who measure urine albumin or protein the most prevalent method is immunoturbidimetric method (12/14) for urine albumin and colorimetric method for urine protein (16/22), respectively. There is a large heterogeneity among type of sample recommended for measuring urine albumin or protein and reporting units, consequently. Survey results are summarized in Table 4.
Table 4. Description of albuminuria/proteinuria assessment regarding preferred sample type, reporting units and reference intervals (or cut-offs) in Croatian medical biochemistry laboratories (questions 10,11,12,20,21,22,23).*
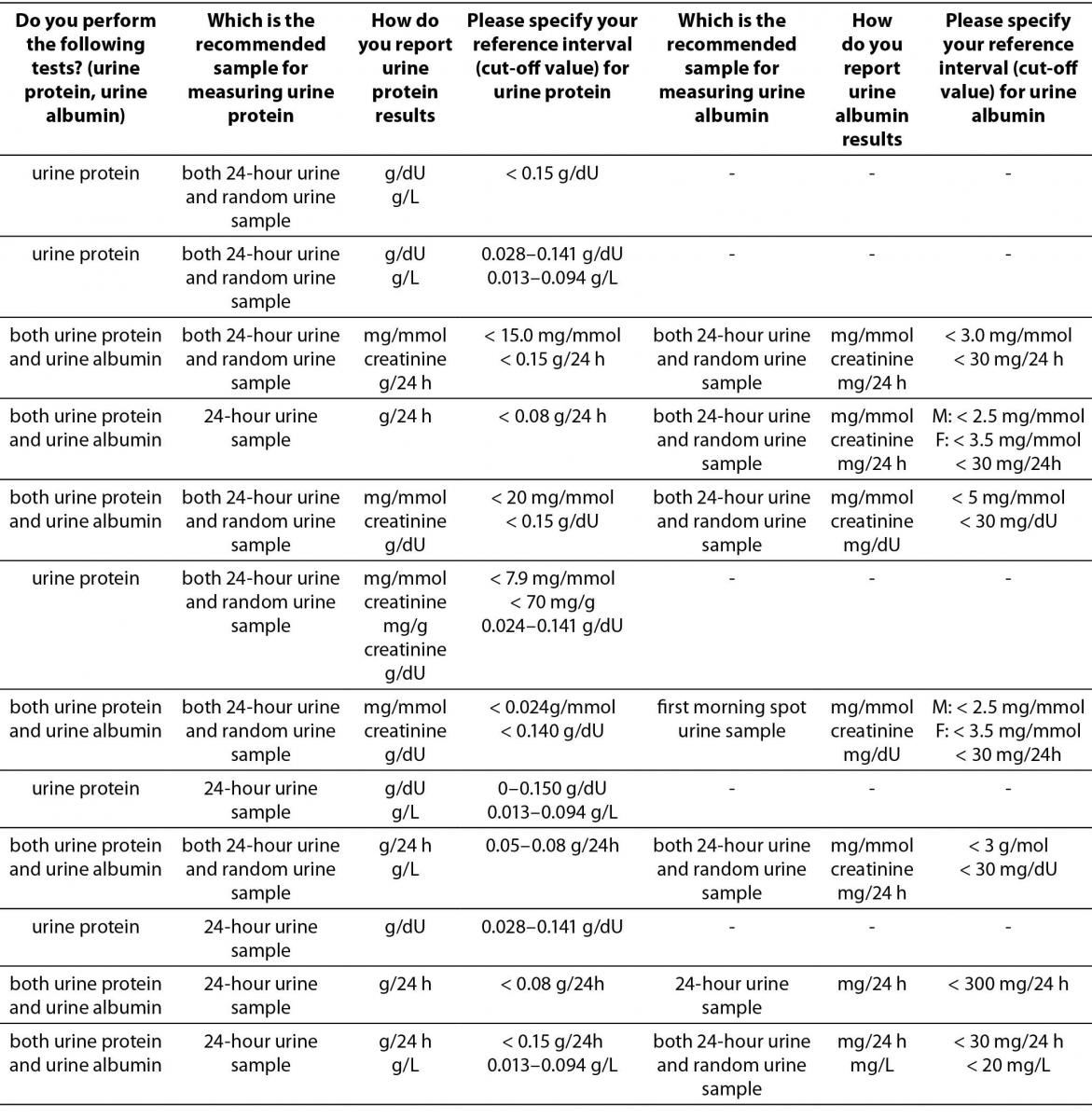
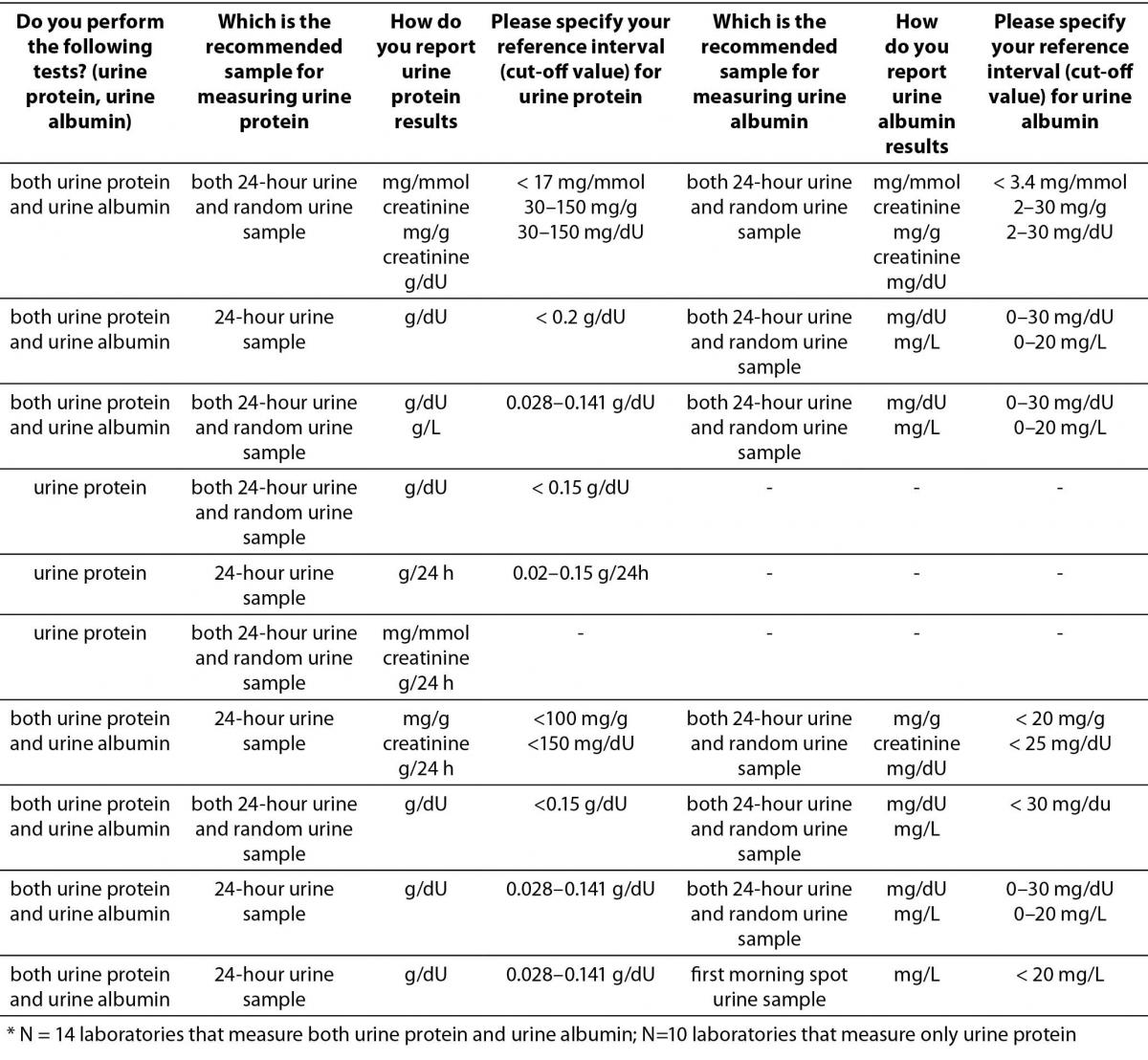
The results indicate that assessment of albuminuria and proteinuria in a large number of laboratories is still performed in 24-hour urine samples. Predominant manufacturer of reagents used for measuring urine albumin or protein is Beckman Coulter (14/22 for urine protein, and 8/14 for urine albumin).
Additional education
76 (out of 80) responders declared their interest in further education in the field of implementation of clinical guidelines into every day routine practice.
Discussion
This is the first study to give insight about laboratory diagnostics of chronic kidney disease in Croatian medical biochemistry laboratories. The motive for this study was the new updated KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease in which the role of laboratory medicine is of great importance. The key prerequisite for the screening of CKD are two simple laboratory tests: eGFR and assessment of albuminuria (or proteinuria) (5). The American Society of Nephrology strongly recommends all adults to undergo routine screening for CKD and some data suggest that screening for CKD is cost-effective in patients with diabetes and hypertension (6,7). However, there are no national Croatian guidelines regarding this area of laboratory medicine, as in some other countries throughout the world (2,3,8,9). This study describes current status of harmonization of creatinine and albuminuria (proteinuria) measurement in Croatian medical biochemistry laboratories.
Harmonization process regarding creatinine standardization in Croatia started in 2010 with the recommendations for revision of creatinine methods and reference intervals published by CCMB (4). This initial process was followed by a course about harmonization in laboratory medicine in 2011 (10). However, it is obvious from the survey results that creatinine standardization process in Croatian laboratories is not finished and only minority of laboratories automatically report eGFR values. The biggest problem raised from this survey results is a fact that there is discrepancy between methods used for creatinine measurement and creatinine reference intervals, i.e. reference intervals for standardized creatinine were used for creatinine results measured by non-standardized methods, and vice versa. Similar problem was observed with equations used for calculating eGFR. Also, reporting values for eGFR are not as recommended (all values greater than 60 mL/min/1.73 m2 report as „> 60 mL/min/1.73 m2“, but are given as the exact calculated number for MDRD equation). Very small number of laboratories uses CKD-EPI equation recommended by KDIGO guideline for calculating eGFR. These results can have serious implications on patient health and there is a need for better regulation in this area of laboratory work. One of the possible approaches can be through pre- and post-analytical modules of Croatian national external quality assessment scheme which is in the domain of Croatian Centre for Quality Assessment in Laboratory Medicine (CROQALM).
Survey results about biomarkers of kidney damage show even higher heterogeneity among Croatian laboratories. There are no recommendations about preferred type of urine sample for an initial screening, and large part of laboratories perform analyses in 24-hour urine samples, regardless of all known disadvantages (11). There is a high variability of reporting units; some laboratories still report absolute concentrations of urine albumin, regardless of the type of urine sample, although there were some efforts made in the area of laboratory diagnostics of diabetic nephropathy to standardize type of samples and reporting units used for measuring urine albumin (12). One of the major problems is the fact that laboratories in primary health care institutions do not measure urine albumin or protein at all.
Some of these problems were acknowledged at a course of laboratory diagnostics of chronic kidney disease held in 2013. However this survey shows that in spite of numerous education efforts from our national associations, the policies and practices concerning CKD in Croatian laboratories are neither uniform nor harmonized (13). For this reason we concluded that education must start at the beginning and provide simple and understandable recommendations and guidelines for initiative screening of CKD followed by comprehensive education through our national associations. Such conclusion is supported by a high number of responders that stated they are interested in further education in the field of implementation of clinical guidelines into everyday routine practice. However, the most troubling fact is that all 4 survey participants that declared against further education, had discrepancy between serum creatinine measurement methods and used reference intervals. One of the goals of this study is to raise awareness amongst Croatian laboratories about critical points in this area of laboratory medicine.
This study has several limitations, similar to those observed in a study conducted by Dukić and Šimundić (14): the survey was self reported and participants might not be providing the accurate and reliable information about the real situation; there was a low number of responders and majority were from primary health care institutions.
Conclusions
It is evident from the survey results that laboratory diagnostics of chronic kidney disease in Croatia is not standardized. Laboratories still use non-standardized methods for creatinine measurement and they do not report eGFR values. Most laboratories still measure urine albumin or protein in 24-hour urine sample, which is also the only recommended sample in some laboratories. There is a large heterogeneity in reference intervals and types of units in albuminuria (or proteinuria) measurement. It is essential to provide a framework for nation-wide harmonization and standardization of laboratory procedures in this area of laboratory medicine.
Acknowledgments
The authors wish to thank Assist. Prof. Jasenka Wagner, Chair of Committee for information and public relations, for assistance in gathering information through this survey and Dr. Graham Jones, Chair of the integrated project of International Federation of Clinical Cheimistry and Laboratory Medicine (IFCC) and World Association of Societies of Pathology and Laboratory Medicine (WASPaLM) Task Force on Chronic Kidney Disease (TF-CKD) for the assistance in the revision of survey questions.


