Introduction
Adiponectin is a protein exclusivelysecreted by mature adipocytes, which circulates in high concentrationsin the blood (1,2). The circulating concentrations of adiponectin are inversely proportional to adiposity, and low adiponectin concentrations predict the development of type 2 diabetes and cardiovascular disease (2). Serumadiponectin levels are influenced by several factors, such as age, sex, body mass index and diabetes (2-5) and are decreased in patients with type 2 diabetes mellitus or metabolic syndrome (5,6). In obese men, serum and interstitial total adiponectin levels are lower than in lean subjects (7). After hyperinsulinemia, serum and interstitial total adiponectin is reduced in both obese and lean men. Current biochemical and physiological evidence suggest that low adiponectin levels may be a consequence of insulin resistance with compensatory hyperinsulinemia (1).
Testosterone is another factor that influences concentration of circulating adiponectin. In healthy subjects, adiponectin production is inhibited by testosterone (2). However, whether testosterone is also associated with circulating adiponectin in patients with diabetes is not very well understood.
In school-aged girls with type 1 diabetes, the decrease in adiponectin concentrations was correlated with increasing levels of testosterone (8). In middle-aged patients with metabolic syndrome, blood concentrations of testosterone were positively correlated witj adiponectin (9). In addition, testosterone therapy in combination with diet and exercise in type 2 diabetic men resulted in an increase of adiponectin (10). To date, there is little information about the association between circulating testosterone and adiponectin in adult diabetic patients. Therefore, we performed this prospective study to investigate the relationship between plasma testosterone and adiponectin in a group of adult patients with type 2 diabetes.
Materials and methods
Patient selection
This study was approved by the institution review board of Liaocheng People’s Hospital. Written informed consent was obtained from all participants. Between January and July 2008, 65 patients with type 2 diabetes were recruited to this study. There were 39 males and 26 females, with an average age of 58 years (range 37-84 years). Twenty one (32.3%) of these patients had concurrent coronary artery disease (CAD), and 22 (33.8%) had renal dysfunction. CAD was confirmed by coronary angiogram and electrocardiogram (ECG) as well as clinical symptoms. Renal dysfunction was established by assessing glomerular filtration rate (GFR < 90 mL/min), which was calculated from serum creatinine (Cr) concentration as follows:
Cockcroft-Gault GFR = (140-age) x (Weight in kg)
x (0.85 if female) / (72 x Cr).
Twenty healthy subjects (Table 1) were also recruited as the control group. These subjects were recruited from the health-check clinics of our hospital. They had no previous medical history of diabetes, cardiovascular or renal disease. Physical examination, chest X-ray, electrocardiogram and laboratory tests for blood glucose, lipids, renal and liver function revealed no abnormalities.
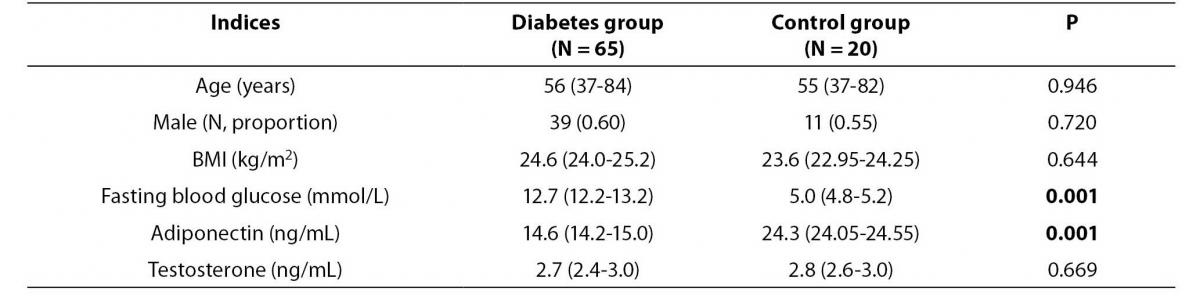
Table 1. Baseline characteristics and serum levels of adiponectin and testosterone of the study and control group.
Biochemical analyses
Five milliliters of blood was collected from the median cubital veinin a sitting position with avacuum tube by the investigators in the mornings between 7-8 am, after overnight fasting. Serum was stored in a freezer at -20°C for up to four weeks until testosterone and adiponectin were analyzed. Biochemical data wereassayed as follows: fasting blood glucose (glucose-oxidase electrode method); total cholesterol (cholesterol oxidase enzymatic assay method);triglyceride (enzymatic colorimetric method); serum creatinine (Jaffe reaction method). The above analyses were conducted by using a fully automated Olympus AU2700 chemistry analyzer (Olympus CO Ltd., Tokyo, Japan). Olympus Life and Material Science Europa GmbH reagents (Hamburg, Germany) were used.
Serum testosterone was determined by the radio-immunoassay(Northern Biotech Inc, Beijing, China). Our laboratory intra- andinter-assay coefficients of variation were less than 6.0% based on 10 measurements using control samples at level of 2.8 ng/L. Total serum adiponectin levels weredetermined by an enzyme-linked immunosorbent assay kit (ELISA) (Shiruike Inc, Shanghai, China) as accordingto the manufacturer’s instructions. The sensitivity of the assay wascalculated to be 1 ng/mL. Our laboratory intra- and inter-assay coefficientsof variation were less than 8% based on 10 measurements using control samples at level of 24.5 ng/L.
Statistical analysis
Data are presented as median and interquartile range (Q1-Q3), except age which was presented as median and range (maximum-minimum). Comparison of variablesbetween groupswas performed by Mann-Whitney test. Kruskal-Wallis one-way analysis of variance was used for three group comparisons. Post hoc analysis was performed by Dunn’s post test. Correlation analysis was done by Spearman’s rank correlation. SPSS software (version 11.5) was used for the statistical analysis. P values less than 0.05 were considered statistically significant.
Results
As shown in Table 1, there were no significant differences in the age and sex between the study and control groups. The mean testosterone concentration did not differ significantly between two groups. However, patients with type 2 diabetes had significantly lower adiponectin concentration than patients in the control group (14.6 (14.2-15.0) vs. 24.3 (24.05-24.55) ng/mL, P = 0.001).
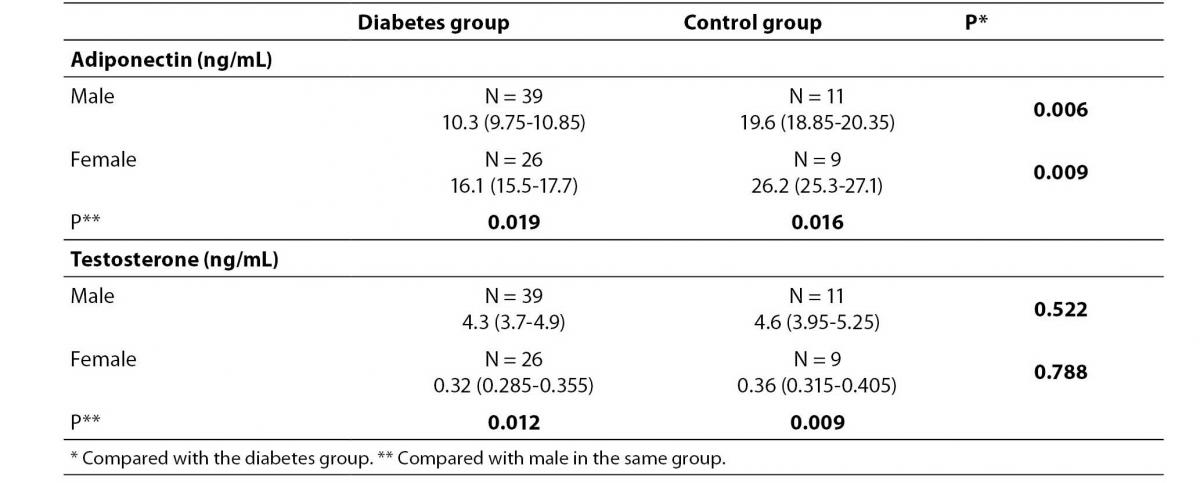
Levels of adiponectin and testosterone according to gender in diabetes and control patient groups are presented in table 2. In both, patient and control group, serum adiponectin concentration was lower in males (P= 0.019 and 0.026, respectively), whereas the average testosterone concentration was higher in males (P= 0.012 and 0.009, respectively) than in females. Adiponectin concentration in males and females of the diabetes group was lower than in males and females of the control group (P= 0.008 and 0.009, respectively).
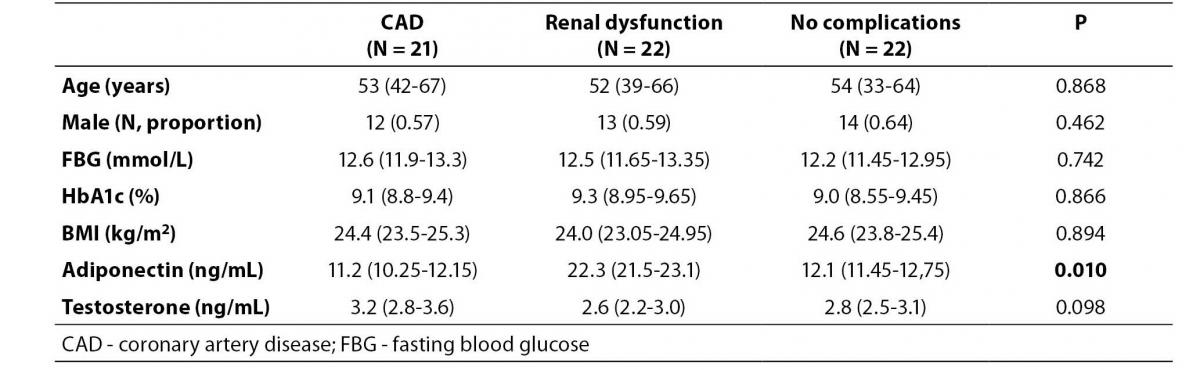
Table 3 shows adiponectin and testosterone levels in patients with and without CAD or renal dysfunction. There were no significant differences in age, gender, blood glucose, BMI or serum testosterone concentration across three groups. However, the serum adiponectin level in the renal dysfunction group differed from the other groups (P = 0.016). Post hoc analysis showed that the median level of adiponectin in the renal dysfunction group was higher than the CAD and non-complication group (P = 0.009).
Table 2. Serum levels of adiponectin and testosterone of the diabetes and control group.
Table 3. Comparison of adiponectin levels between diabetic patients with and without cardiovascular complications.
Univariate correlation analysis showed an inverse weak correlation between adiponectin and testosterone levels in male diabetic patients (r = -0.27, P= 0.009). There was no significant correlation between adiponectin and testosterone in female patients (r = -0.05, P = 0.167). Also, no significant correlation between serum adiponectin and testosterone was found in the healthy subjects, when male (r = -0.06, P = 0.412) and female (r = -0.09, P = 0.225) subjects were analyzed either separately or as a unique group (r = -0.06, P = 0.165). An inverse weak correlation between BMI and adiponectin was also found in the diabetes group (r = -0.33, P= 0.02).
Discussion
The major findings of this study are: 1) serum adiponectin concentration in patients with type 2 diabetes was significantly higher than in controls; 2) serum level of adiponectin in patients with renal dysfunction was higher than in those with normal renal function; 3) there was an inverse weak correlation between serum adiponectin level and BMI or serum testosterone.
The results of our study were consistent with the previous studies where a lower serum adiponectin in diabetics than in healthy subjects was revealed (6,8). A recent population study showed that high serum adiponectin levels were associated with a lower incidence of type 2 diabetes (11).This relationship was more significant in female subjects than in males.The authors of this study concluded that the baseline adiponectin levels play a protective role in the pathogenesis of diabetes and are independent predictors for diabetes.The level of adiponectin seems to be closely associated with gender of the healthy subjects. The median serum levels of adiponectin in women was found to be 50% higher than in men (12). In addition, serum levels of adiponectin are also related to age. Older age is associated with a higher adiponectin level in both men and women (12). In our study we also found that in the healthy subjects the adiponectin level in women was higher than in men. Perhaps more importantly, the serum adiponectin levels in male diabetic patients were lower than in the female patients. These results suggest that adiponectin as a cardiovascular protector has diminished more in male diabetic patients.
The lower level of adiponectin in male diabetic patients may be due to a number of factors but a higher concentration of serum testosterone is likely one of them. An inverse weak correlation was found between adiponectin and testosterone concentration in the male patients. Iniguez and colleagues (8) recently measured the serum adiponectin and testosterone in 56 pubertal girls with type 1 diabetes. They found that decreasing adiponectin levels observed in these adolescents with type 1 diabetes correlate with increasing testosterone levels. However, the mechanisms by which testosterone affected the adiponectin levels were unclear (8).
From numerous studies it was suggested that adiponectin constitutesan important protective factor in the pathogenesis of the metabolicsyndrome and cardiovascular disease by exerting antidiabeticand antiatherogenic actions (13). Diminished serum adiponectinconcentrations, on the other hand, are predictive for the developmentof diabetes and cardiovascular disease (14).Testosterone also plays a role in the pathogenesis of type 2 diabetes. Serum testosterone was reduced in male patients with type 2 diabetes, whereas it was increased in the female patients (15). It was believed that testosterone reduces the risk of type 2 diabetes in men but increases the risk in women (15). Given the antidiabeticand antiatherogenic actions of adiponectin and the potential antidiabetic actions of testosterone in men, reduced adiponectin and testosterone concentrations in males mayat least in part account for the higher incidence of cardiovasculardisease in men due to a diminished protective effect of these endogenous hormones.
In this study, the serum adiponectin level in patients with CAD did not differ from those without CAD (Table 3).This may be due to the fact that the pathogenesis of CAD in our patients may be multi-factorial, involving other risk factors, such as blood plasma lipoproteins, high blood pressure etc., which were not explored in detail in this study.The adiponectin level in patients with renal dysfunction, however, was significantly higher than in those without renal dysfunction.Previous studies have suggested thata decrease in adiponectin clearance in the kidney may increase levels of adiponectin, and adiponectin seems to be influenced more strongly by renal function than by sex hormones (12). In diabetic patients, the adiponectin levels were increased and were closely associated with the glomerular filtration rate, or with the stages of renal insufficiency (16-18). Although correlation analysis between adiponectin and renal function was not performed in our study, the reduced renal clearance was probably the main cause of increased serum adiponectin.
A potential limitation of this study is that the number of patients (N = 65) is relatively small. Whether a larger patient population would yield a stronger correlation between plasma testosterone and adiponectin in male and female patients remains to be seen. In addition, majority of studies have shown that testosterone is significantly lower in men with type 2 diabetes than in women (19). In our study, the mean value of plasma testosterone in men was higher than in women. Whether this is due to the small number of patients needs further investigation.
In summary, this study has confirmed that the levels of serum adiponectin in adult patients with type 2 diabetes are lower than in the healthy subjects. An inverse correlation between serum adiponectin and testosterone has also been demonstrated in male diabetic patients.
Notes
Potential conflict of interest
None declared.
References
1. Cook JR, Semple RK. Hypoadiponectinemia--cause or consequence of human “insulin resistance”? J Clin Endocrinol Metab 2010;95:1544-54.
2. Swarbrick MM, Havel PJ. Physiological, pharmacological, and nutritional regulation of circulating adiponectin concentrations in humans. Metab Syndrome Related Disorder 2008;6:87-102.
3. Gavrila A, Chan JL, Yiannakouris N, Kontogianni M, Miller LC, Orlova C, et al. Serum adiponectin levels are inversely associated with overall and central fat distribution but are not directly regulated by acute fasting or leptin administration in humans: cross-sectional and interventional studies. J Clin Endocrinol Metab 2003;88:4823-31.
5. Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol 2000;20:1595-9.
6. Kapoor D, Clarke S, Stanworth R, Channer KS, Jones TH. The effect of testosterone replacement therapy on adipocytokines and C-reactive protein in hypogonadal men with type 2 diabetes. Eur J Endocrinol 2007;156:595-602.
7. Murdolo G, Hammarstedt A, Schmelz M, Jansson PA, Smith U. Acute hyperinsulinemia differentially regulates interstitial and circulating adiponectin oligomeric pattern in lean and insulin-resistant, obese individuals. J Clin Endocrinol Metab 2009;94:4508-16.
8. Iniguez G, Torrealba MI, Avila A, Cassorla F, Codner E. Adiponectin serum levels and their relationships to androgen concentrations and ovarian volume during puberty in girls with type 1 diabetes mellitus. Hormone Res 2008;70:112-7.
9. Akishita M, Fukai S, Hashimoto M, Kameyama Y, Nomura K, Nakamura T, et al. Association of low testosterone with metabolic syndrome and its components in middle-aged Japanese men. Hypertension Res 2010;33:587-91.
10. Heufelder AE, Saad F, Bunck MC, Gooren L. Fifty-two-week treatment with diet and exercise plus transdermal testosterone reverses the metabolic syndrome and improves glycemic control in men with newly diagnosed type 2 diabetes and subnormal plasma testosterone. J Androl 2009;30:726-33.
11. Snijder MB, Heine RJ, Seidell JC, Bouter LM, Stehouwer CD, Nijpels G, et al. Associations of adiponectin levels with incident impaired glucose metabolism and type 2 diabetes in older men and women: the Hoorn study. Diabetes Care 2006;29:2498-503.
12. Isobe T, Saitoh S, Takagis, Takeuchi H, Chiba Y, Katoh N, Shimamoto K. Influence of gender, age and renal function on plasma adiponectin level: the Tanno and Sobetsu Study. Eur J Endocrinol 2005;153:91-8.
13. Matsuzawa Y, Funahashi T, Kihara S, Shimomura I. Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol 2004;24:29-33.
14. Spranger J, Kroke A, Mohlig M, Bergmann MM, Ristow M, Boeing H, et al. Adiponectin and protection against type 2 diabetes mellitus. Lancet 2003;361:226-8.
15. Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2006;295:1288-99.
16. Schalkwijk CG, Chaturvedi N, Schram MT, Fuller JH, Stehouwer CD. EURODIAB Prospective Complications Study Group. Adiponectin is inversely associated with renal function in type 1 diabetic patients. J Clin Endocrinol Metab 2006;91:129-35.
17. Komaba H, Igaki N, Goto S, Yokota K, Doi H, Takemoto T, et al. Increased serum high-molecular-weight complex of adiponectin in type 2 diabetic patients with impaired renal function. Am J Nephrol 2006;26:476-82.
18. Saraheimo M, Forsblom C, Fagerudd J, Teppo AM, Pettersson-Fernholm K, Frystyk J, et al. Serum adiponectin is increased in type 1 diabetic patients with nephropathy: FinnDiane study group. Diabetes Care 2005;28:1410-4.
19. Maric C, Sex, diabetes and the kidney. Am J Physiol- Renal Physiol 2009;296:F680-8.