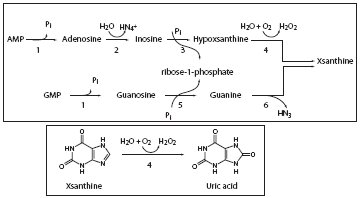
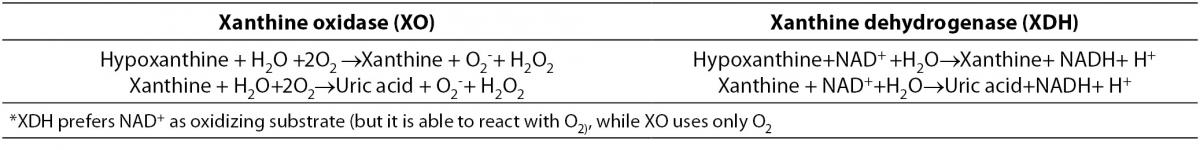
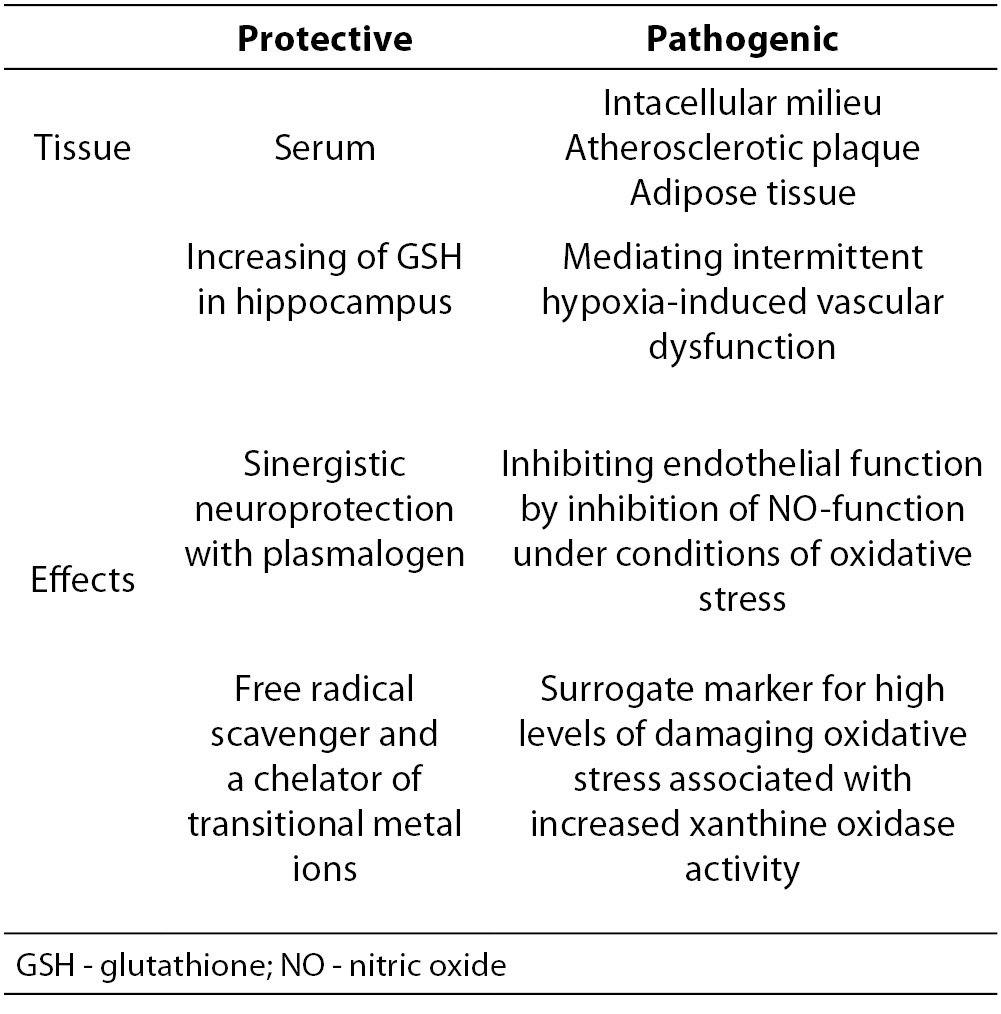
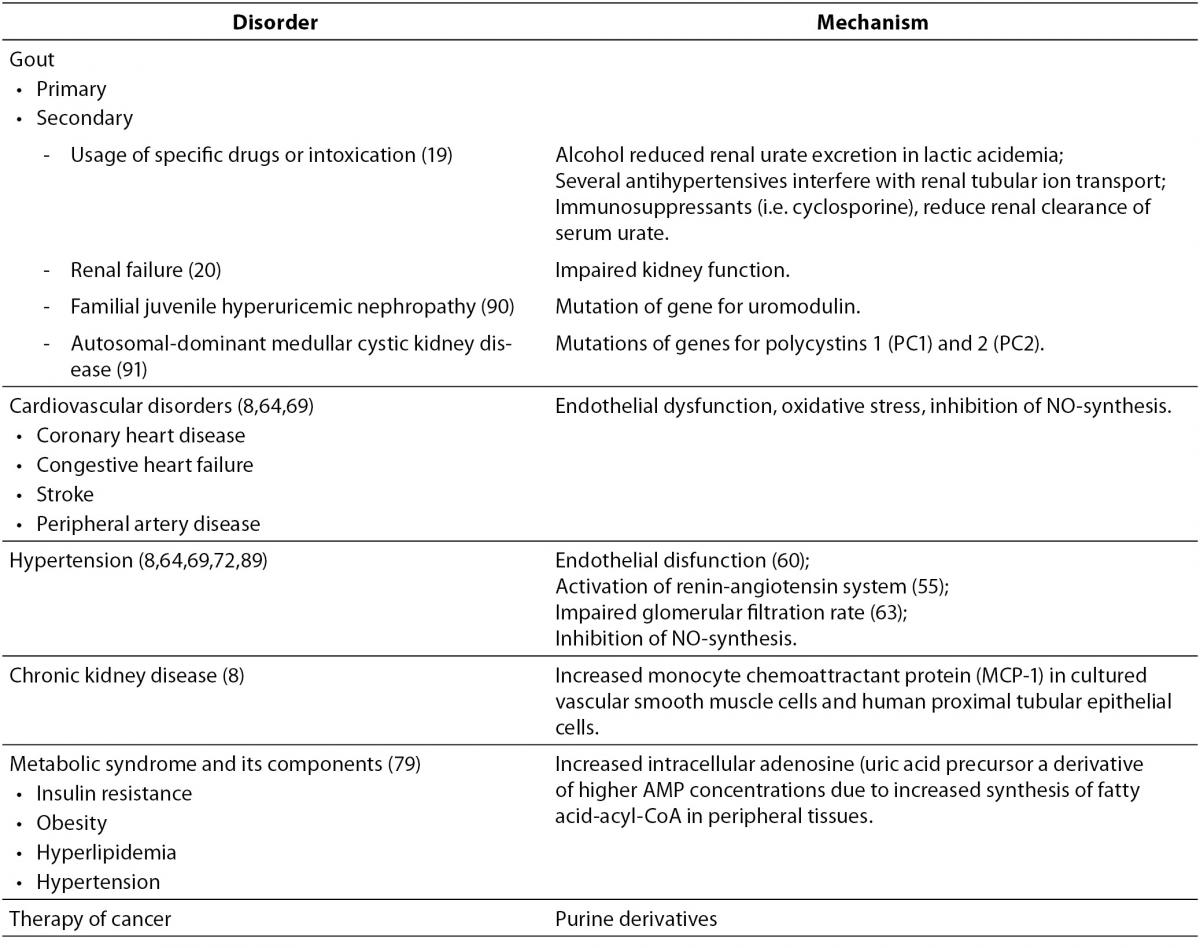
References
1. George J, Struthers AD. Role of urate, xanthine oxidase and the effects of allopurinol in vascular oxidative stress. Vasc Health Risk Manag 2009;5:265-72.
2. Benedict JD, Forsham PH, Stetten D, Jr. The metabolism of uric acid in the normal and gouty human studied with the aid of isotopic uric acid. J Biol Chem 1949;181:183-93.
3. Rodwell VW. Metabolism of purine and pyrimidine nucleotides. In: Murray RK, Bender DA, Botham KM, Kennelly PJ, Rodwell VW, Weil PA, eds. Harper’s Illustrated Biochemistry. New York: McGraw Hill; 2009. p. 292-301.
4. Pacher P, Nivorozhkin A, Szabo C. Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev 2006;58:87-114.
5. Chilappa CS, Aronow WS, Shapiro D, Sperber K, Patel U, Ash JY. Gout and hyperuricemia. Compr Ther 2010;36:3-13.
6. Halabe, A, Sperling O. Uric acid nephrolithiasis. Miner Electrolyte Metab 1994;20:424-31.
7. Baker JF, Krishnan E, Chen L, Schumacher HR. Serum uric acid and cardiovascular disease: recent developments, and where do they leave us? Am J Med 2005;118:816-26.
8. Kanbay M, Solak Y, Dogan E, Lanaspa MA, Covic A. Uric acid in hypertension and renal disease: the chicken or the egg? Blood Purif 2010;30:288-95.
9. Maalouf NM. Metabolic syndrome and the genesis of uric acid stones. J Ren Nutr 2011;21:128-31.
10. Waring WS. Uric acid: an important antioxidant in acute ischaemic stroke. Q J Med 2002;95:691-3.
11. Ferguson LD, Walters MR. Xanthine oxidase inhibition for the treatment of stroke disease: a novel therapeutic approach Expert Rev Cardiovasc Ther 2011;9:399-401.
12. Newman DJ, Price CP. Nonprotein Nitrogen Metabolites. In: Burtis CA, Ashwood ER. Eds. Tietz Fundamentals of Clinical Chemistry. Philadelphia: Saunders; 2001. p.414-26.
13. Terkeltaub R, Bushinsky DA, Becker MA. Recent developments in our understanding of the renal basis of hyperuricemia and the development of novel antihyperuricemic therapeutics. Arthritis Research & Therapy 2006;8 Suppl 1:S4.
14. Watanabe S, Kang DH, Feng L, Nakagawa T, Kanellis J, Lan H, et al. Uric acid, hominoid evolution, and the pathogenesis of salt-sensitivity. Hypertension 2002;40:355-60.
15. Wu XW, Lee CC, Muzny DM, Caskey CT. Urate oxidase: primary structure and evolutionary implications. Proc Natl Acad Sci USA 1989;86:9412-6.
16. Liebman SE, Taylor JG, Bushinsky DA. Uric Acid Nephrolithiasis. Curr Rheumatol Rep 2007;9:251-7.
17. Hak AE, Choi HK. Menopause, postmenopausal hormone use and serum uric acid levels in US women – the Third National Health and Nutrition Examination Survey. Arthritis Res Ther 2008;10:R116.
18. Richette P, Bardin T. Gout. Lancet 2010;375:318-28.
19. Schlesinger N. Diagnosis of Gout: Clinical, Laboratory, and Radiologic findings. Am J Manag Care 2005;11(15 Suppl):S443-50; quiz S465-8.
20. Gomis Couto A, Teruel Briones JL, Fernández Lucas M, Rivera Gorrin M, Rodríguez Mendiola N, Jiménez Álvaro S, Quereda Rodríguez-Navarro C. Causes of unplanned hemodialysis initiation. Nefrologia 2011;31:733-7.
21. Bleyer AJ. Improving the recognition of hereditary interstitial kidney disease. J Am Soc Nephrol 2009;20:11-3.
22. Pillinger MH, Goldfarb DS, Keenan RT. Gout and its comorbidities. Bull NYU Hosp Jt Dis 2010;68:199-203.
23. Neogi T. Clinical practice. Gout. N Engl J Med 2011;364:443-52.
24. Becker MA, Schumacher HR, Espinoza LR, Wells AF, MacDonald P, Lloyd E, Lademacher C. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial. Arthritis Res Ther 2010;12:R63.
25. Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer 2003;3:276-85.
26. Schulz E, Gori T, Münzel T. Oxidative stress and endothelial dysfunction in hypertension. Hypertens Res 2011;34:665-73.
27. Riegersperger M, Covic A, Goldsmith D. Allopurinol, uric acid, and oxidative stress in cardiorenal disease. Int Urol Nephrol 2011;43:441-9.
28. Harrison R. Structure and function of xanthine oxidoreductase: where are we now? Free Radic Biol Med 2002;33:774-97.
29. Nabipour I, Sambrook PN, Blyth FM, Janu MR, Waite LM, Naganathan V, et al. Serum uric acid is associated with bone health in older men: A cross-sectional population-based study. J Bone Miner Res 2011;26:955-64.
30. Strazzullo P, Puig JG. Uric acid and oxidative stress: relative impact on cardiovascular risk? Nutr Metab Cardiovasc Dis 2007;17:409-14.
31. Saliaris AP, Amado LC, Minhas KM, Schuleri KH, Lehrke S, St John M, et al. Chronic allopurinol administration ameliorates maladaptive alterations in Ca2+ cycling proteins and β-adrenergic hyporesponsiveness in heart failure. Am J Physiol Heart Circ Physiol 2007;292:H1328-35.
32. Dopp JM, Philippi NR, Marcus NJ, Olson EB, Bird CE, Moran JJ, et al. Xanthine Oxidase Inhibition Attenuates Endothelial Dysfunction Caused by Chronic Intermittent Hypoxia in Rats. Respiration 2011;82:458-67.
33. Yamazaki I, Soma T, Ichikawa Y, Iwai Y, Kondo J, Matsumoto A. Usefulness of allopurinol for prevention of myocardial reperfusion injury in open heart surgery. Nippon Kyobu Geka Gakkai Zasshi 1995;43:26-31.
34. Daly JW. Caffeine analogs: biomedical impact. Cell Mol Life Sci 2007;64:2153-69.
35. Szentmiklósi AJ, Cseppentō A, Gesztelyi R, Zsuga J, Körtvély A, Harmati G, Nánási PP. Xanthine derivatives in the heart: blessed or cursed? Curr Med Chem 2011;18:3695-706.
36. Okafor C, Liao R, Perreault-Micale C, Li X, Ito T, Stepanek A, et al. Mg-ATPase and Ca2+ activated myosin ATPase activity in ventricular myofibrils from non-failing and diseased human hearts – effects of calcium sensitizing agents MCI-154, DPI 201 – 106, and caffeine. Mol Cell Biochem 2003;245:77-89.
37. Gersch C, Palii SP, Kim KM, Angerhofer A, Johnson RJ, Henderson GN. Inactivation of nitric oxide by uric acid. Nucleosides Nucleotides Nucleic Acids. 2008;27:967-78.
38. Wei L, Mackenzie IS, Chen Y, Struthers AD, MacDonald TM. Impact of allopurinol use on urate concentration and cardiovascular outcome. Br J Clin Pharmacol 2011;71:600-7.
39. Rajendra NS, Ireland S, George J, Belch JJ, Lang CC, Struthers AD. Mechanistic insights into the therapeutic use of high-dose allopurinol in angina pectoris.J Am Coll Cardiol 2011;58:820-8.
40. Bergamini C, Cicoira M, Rossi A, Vassanelli C. Oxidative stress and hyperuricaemia: pathophysiology, clinical relevance, and therapeutic implications in chronic heart failure. Eur J Heart Fail 2009;11:444-52.
41. Nieto FJ, Iribarren C, Gross MD, Comstock GW, Cutler RG. Uric acid and serum antioxidant capacity: a reaction to atherosclerosis? Atherosclerosis 2000;148:131-9.
42. Glantzounis GK, Tsimoyiannis EC, Kappas AM, Galaris DA. Uric acid and oxidative stress. Curr Pharm Des 2005;11: 4145e51.
43. Reunanen A, Takkunen H, Knekt P, Aromaa A. Hyperuricemia as a risk factor for cardiovascular mortality. Acta Med Scand Suppl 1982;668:49-59.
44. Bonora E, Targher G, Zenere MB, Saggiani F, Cacciatori V, Tosi F, et al. Relationship of uric acid concentration to cardiovascular risk factors in young men. Role of obesity and central fat distribution. The Verona Young Men Atherosclerosis Risk Factors Study. Int J Obes Relat Metab Disord 1996;20:975-80.
45. Lippi G. Relationship between uric acid, hyperglycemia and hypertriglyceridemia in general population. Biochem Med 2008;18:37-41.
46. Fang J, Alderman MH. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971–1992: National Health and Nutrition Examination Survey. JAMA 2000;283:2404-10.
47. Kaya EB, Yorgun H, Canpolat U, Hazırolan T, Sunman H, Ülgen A, Ates AH, et al. Serum uric acid levels predict the severity and morphology of coronary atherosclerosis detected by multidetector computed tomography. Atherosclerosis 2010;213:178-83.
48. Krishnan E, Pandya BJ, Chung L, Dabbous O. Hyperuricemia and the risk for subclinical coronary atherosclerosis - data from a prospective observational cohort study. Arthritis Res Ther 2011;13:R66.
49. Strasak A, Ruttmann E, Brant L, Kelleher C, Klenk J, Concin H, et al. Serum Uric Acid and Risk of Cardiovascular Mortality: A Prospective Long-Term Study of 83 683 Austrian Men. Clin Chem 2008;54:273-84.
50. Pascual-Figal DA, Hurtado-Martínez JA, Redondo B, Antolinos MJ, Ruiperez JA, Valdes M. Hyperuricaemia and long-term outcome after hospital discharge in acute heart failure patients. Eur J Heart Fail 2007;9:518-24.
51. Lim HE, Kim SH, Kim EJ, Kim JW, Rha SW, Seo HS, Park CG. Clinical value of serum uric Acid in patients with suspected coronary artery disease. Korean J Intern Med 2010;25:21-6.
52. Culleton BF, Larson MG, Kannel WB, Levy D. Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med 1999;131:7-13.
53. Neogi T, Terkeltaub R, Ellison RC, Hunt S, Zhang Y. Serum Urate Is Not Associated with Coronary Artery Calcification: The NHLBI Family Heart Study. J Rheumatol 2011;38:111-7.
54. Alcaíno H, Greig D, Castro P, Verdejo H, Mellado R, García L, et al. The role of uric acid in heart failure. Rev Med Chile 2011;139:505-15.
55. Proctor PH. Uric Acid and Neuroprotection. Stroke 2008;39: e126.
56. Dawson J, Quinn TJ, Walters MR. Response to Letter by Proctor. Stroke 2008;39:e89.
57. Proctor PH. Uric acid: neuroprotective or neurotoxic? Stroke. 2008;39:e88.
58. Aoyama K, Matsumura N, Watabe M, Wang F, Kikuchi-Utsumi K, Nakaki T. Caffeine and uric acid mediate glutathione synthesis for neuroprotection. Neuroscience 2011;181:206-15.
59. Amaro S, Planas AM, Chamorro A. Uric acid administration in patients with acute stroke: a novel approach to neuroprotection. Expert Review of Neurotherapeutics 2008;8:259-70.
60. Amaro S, Chamorro A. Translational stroke research of the combination of thrombolysis and antioxidant therapy. Stroke 2011;42:1495-9.
61. Doehner W, von Haehling S, Anker SD. Uric acid as a prognostic marker in acute heart failure—new expectations from an old molecule. Eur J Heart Fail 2007;9:437-9.
62. Gruson D, Ahn SA, Rousseau MF. Biomarkers of inflammation and cardiac remodeling: the quest of relevant companions for the risk stratification of heart failure patients is still ongoing. Biochem Med 2011;21:254-63.
63. Manzano L, Babalis D, Roughton M, Shibata M, Anker SD, Ghio S, et al.; SENIORS Investigators. Predictors of clinical outcomes in elderly patients with heart failure. Eur J Heart Fail 2011;13:528-36.
64. Feig DI, Kang DH, Nakagawa T, Mazzali M, Johnson RJ. Uric acid and hypertension. Curr Hypertens Rep 2006;8:111-5.
65. Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA. 2008;300:924-32.
66. Feig DI, Johnson RJ. The role of uric acid in pediatric hypertension. J Ren Nutr 2007;17:79-83.
67. Silverstein DM, Srivaths PR, Mattison P, Upadhyay K, Midgley L, Moudgil A, et al. Serum uric acid is associated with high blood pressure in pediatric hemodialysis patients. Pediatr Nephrol 2011;26:1123-8.
68. Kostka-Jeziorny K, Uruski P, Tykarski A. Effect of allopurinol on blood pressure and aortic compliance in hypertensive patients. Blood Press 2011;20:104-10.
69. Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, et al. Hyperuricemia induces endothelial dysfunction. Kidney Int 2005;67:1739-42.
70. Durante P, Chávez M, Pérez M, Romero F, Rivera F. Effect of uric acid on hypertension progression in spontaneously hypertensive rats. Life Sci 2010;86:957-64.
71. Sautin YY, Nakagawa T, Zharikov S, Johnson. Adverse effects of the classic antioxidant uric acid in adipocytes: ADPH oxidase mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol 2007;93:C584-96.
72. Jung DH, Lee YJ, Lee HR, Lee JH, Shim JY. Association of renal manifestations with serum uric acid in Korean adults with normal uric acid levels. J Korean Med Sci 2010;25:1766-70.
73. Filippatos GS, Ahmed MI, Gladden JD, Mujib M, Aban IB, Love TE, et al. Hyperuricaemia, chronic kidney disease, and outcomes in heart failure: potential mechanistic insights from epidemiological data. Eur Heart J 2011;32:712-20.
74. Kutzing MK, Firestein BL. Altered uric acid levels and disease states. J Pharmacol Exp Ther 2008;324:1-7.
75. Anzai N, Kanai Y, Endou H. New insights into renal transport of urate. Curr Opin Rheumatol 2007;19:151-7.
76. Serpa Neto A, Rossi FM, Valle LG, Teixeira GK, Rossi M. Relation of uric acid with components of metabolic syndrome before and after Roux-en-Y gastric bypass in morbidly obese subjects. Arq Bras Endocrinol Metabol 2011;55:38-45.
77. Otsuki M, Kitamura T, Goya K, Saito H, Mukai M, Kasayama S, et al. Association of urine acidification with visceral obesity and the metabolic syndrome. Endocr J 2011;58:363-7.
78. Lim JH, Kim YK, Kim YS, Na SH, Rhee MY, Lee MM. Relationship between serum uric Acid levels, metabolic syndrome, and arterial stiffness in korean. Korean Circ J 2010;40:314-20.
79. Gil-Campos M, Aguilera CM, Cañete R, Gil A. Uric acid is associated with features of insulin resistance syndrome in obese children at prepubertal stage. Nutr Hosp 2009;24:607-13.
80. Nakagawa T, Hu H, Zharikov S, Tuttle KR, Short RA, Glushakova O, et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol 2006;290:F625-31.
81. Johnson R J, Segal MS, Sautin Y, Nakagawa T, Feig DI, Kang D-H, et al. Potential role of sugar (fructose) in the epidemic of hypertension,obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr 2007;86:899-906.
82. Lê KA, Tappy L. Metabolic effects of fructose. Curr Opin Clin Nutr Metab Care 2006;9:469-75.
83. Tomiyama H, Higashi Y, Takase B, Node K, Sata M, Inoue T, et al. Relationships Among Hyperuricemia, Metabolic Syndrome, and Endothelial Function. Am J Hypertens 2011;24:770-4.
84. Vlasic V, Trifunovic J, Cepelak I, Nimac P, Zrinski Topic R, Dodig S. Urates in exhaled breath condensate of children with obstructive sleep apnea. Biochem Med 2011;21:139-44.
85. Ballesta-Claver J, Díaz Ortega IF, Valencia-Mirón MC, Capitán-Vallvey LF. Disposable luminol copolymer-based biosensor for uric acid in urine. Anal Chim Acta 2011;702:254-6.
86. Barr WG. Uric Acid. Editors In: Walker HK, Hall WD, Hurst JW, editors. Source Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edition. Boston: Butterworths; 1990. Chapter 165.
87. Nishida Y, Iyadomi M, Higaki Y, Tanaka H, Hara M, Tanaka K. Influence of physical activity intensity and aerobic fitness on the anthropometric index and serum uric acid concentration in people with obesity. Intern Med 2011;50:2121-8.
88. Guidi CG, Salvagno GL. Reference intervals as a tool for total quality management. Biochem Med 2010;20:165-72.
89. Johnson RJ, Feig DI, Herrera-Acosta J, Kang DH. Resurrection of uric acid as a causal risk factor in essential hypertension Hypertension 2005;45:18-20.
90. Dahan K, Devuyst O, Smaers M, Vertommen D, Loute G, Poux JM, et al. A cluster of mutations in the UMOD gene causes familial juvenile hyperuricemic nephropathy with abnormal expression of uromodulin. J Am Soc Nephrol 2003;14:2883–93.
91. Dedoussis GV, Luo Y, Starremans P, Rossetti S, Ramos AJ, Cantiello HF, et al. Co-inheritance of a PKD1 mutation and homozygous PKD2 variant: a potential modifier in autosomal dominant polycystic kidney disease. Eur J Clin Invest 2008;38:180-90.