Abnormal gel flotation in a patient with apperant pneumonia diagnosis: a case report
Fethullah Gerin
[*]
[1]
Dilber Coban Ramazan
[1]
Ozgur Baykan
[1]
Onder Sirikci
[1]
Goncagul Haklar
[1]
Introduction
Serum blood collection tubes with separator gel are widely used by many laboratories for chemistry analyses due to the advantage of the barrier gel that facilitates quick and complete separation of serum from blood cells (1). Tubes with separator gel improve serum stability for analysis, diminish sample manipulations, reduce aerosolization of hazardous substances, provide higher serum or plasma level (2), decrease the probability of contamination or sample switching. The use of tubes with separator gel as primary tubes enable their use for storage which eliminates the requirement of re-barcoding also (3). These tubes contain an inert, thixotropic polymer gel as the separator gel. The gravity of this gel is 1.04 g/cm3 to permit its suitable positioning between serum and cellular constituents of blood upon centrifugation. The density of the liquid and cellular components of the blood typically ranges from 1.026 to 1.031 g/cm3 and 1.092 to 1.095 g/cm3, respectively (2,4). Due to differences in density of these components, the gel is displaced and moves upward and forms a barrier between serum and blood cells upon centrifugation and prevents the crossing of molecules and proteins released from the cells to the serum. In some rare situations like hyperproteinemia and administration of a contrast dye, serum or plasma densities increase and abnormal gel flotation may occur (2). Faught et al. carried out a comparison study between different seperator gel tubes. They showed that gel flotation was observed in BD Serum Vacutainer SST tubes between a specific gravity of 1.054-1.057 (5).
Material and methods
Case history
We describe a case of a primary blood collection tube filled with blood sample and a floating separator gel. The blood sample was collected from a 51 years old female in intensive care unit with the diagnosis of pneumonia into a BD Vacutainer SST tube (Becton Dickinson, NJ, USA) containing serum separator gel and conveyed to the core laboratory of Marmara University Pendik R&E Hospital within 30 minutes from collection. Sample was immediately centrifuged at room temperature at 1500 x g for 10 minutes. After centrifugation it was observed that the gel was located at the topmost layer, whereas the serum remained in the middle and the blood cells at the bottom (Figure 1). The specimen was centrifuged for a second time and the gel flotation pattern did not change. This atypical separation pattern suggested that the specific gravity of the serum could be higher than the gel layer. We tried to aspirate the serum under the gel layer by a pipette but we couldn’t, due to the occlusion of pipette tip by the gel. The gross appearance of the serum showed mild hemolysis, but lipemia and icterus were not visibly present. When we checked the information system, we realized that the patient was diagnosed to have pneumonia. Her C-reactive protein was 264.3 mg/L and procalcitonin was 0.84 ng/mL. Additionally, H. Influenza was identified in her sputum culture. A second sample taken from the patient showed the same flotation pattern.
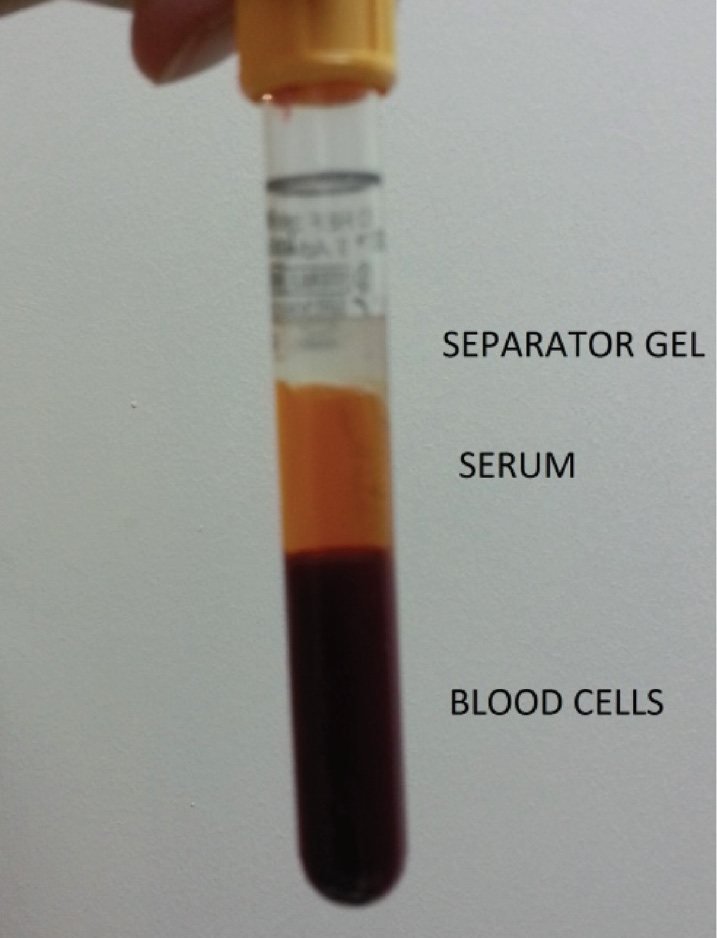
Figure 1. Abnormal floating separator gel.
We couldn’t find another case with abnormal flotation of the gel in patients with pneumonia in the literature.
When consulted, the patient’s physician informed us that the underlying diagnosis of the patient was multiple myeloma, but admitted to the hospital because of pneumonia. A subsequent blood sample was collected from the patient into a plain blood tube and biochemical analyses were performed.
Results
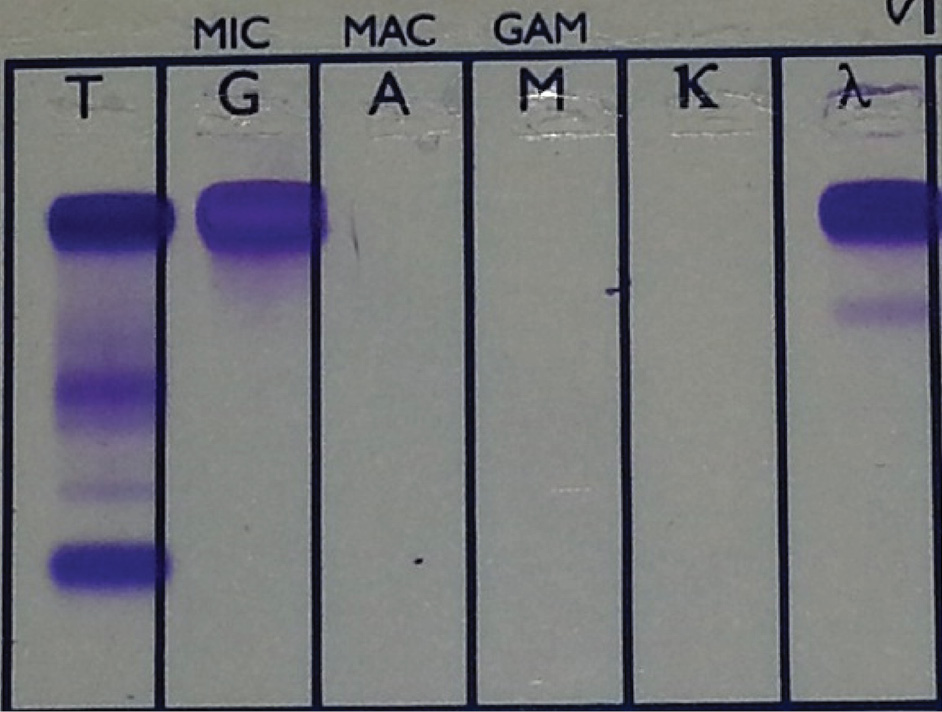
The analyses revealed a highly increased total protein concentration of 145 g/L (reference interval 64-83 g/L). The nephelometric analyses showed a high serum IgG concentration of 108 g/L (reference interval 6.5-16 g/L) and an IgG lambda monoclonal band was determined by serum immunofixation electrophoresis (Helena Biosciences, Europe) (Figure 2).
Figure 2. IgG lambda monoclonal band on serum immunofixation electrophoresis.
Discussion
In some situations the gel in blood collection tubes may be located in abnormal positions. A number of variables can affect the location of the gel. Variables such as specific gravity, yield stress, viscosity, density, and tube material are among the parameters that can be controlled by the manufacturers, whereas centrifugation speed and temperature, acceleration/deceleration during centrifugation processes, and storage conditions can be controlled by the laboratory. However, some patient specific conditions like low hematocrit, increased serum density, anticoagulation therapy, iodinated blood contrast media and increased plasma proteins may cause abnormal gel floatation (6).
Although this abnormal sample was detected visually after centrifugation and before analysis, resampling and retesting had to be done to resolve the case. Since the efforts did not solve the problem, communication with the following physician was required, when underlying multiple myeloma diagnosis was revealed. Whether it is detected by visual inspection or noticed after causing technical problem, the prescence of all relevant information about the patient in the information system would be time and money saving by avoiding resampling, additional testing, and communication efforts with the clinicians.
In similar cases of abnormally placed separating gels, limitation of the gel separator tubes in patients with a high plasma density should be kept in mind and as in this case, since the apparent diagnosis on the hospital information system may not always reveal the underlying problem to its full extent. Therefore contacting the physician in charge may always be more helpful during troubleshooting.
Nowadays, many core laboratories of high volume hospitals use preanalytical automation systems. In these laboratories, these primary collection tubes may be processed without a direct visual inspection by the laboratory technician after automatic centrifugation and it can be difficult to realize gel abnormalities as described. Therefore, these laboratories are vulnerable to occlusion of sample probes from inappropriately separated blood samples. The partial or complete obstruction of sample probes, gel aspiration and/or inadequate sample aspiration by laboratory instrumentation may affect test results, increase turn-around time, and the cost of analyses (4,5,7).
Conclusion
This case report shows that it is very important for the laboratory that the clinical diagnosis of the patient stated in the information system reveal known comorbid conditions besides the apparent admission reason. This case report should also increase our awareness towards the limitation of the separator gel tubes in patients with a high plasma density and its effects on test results and costs of laboratory errors (6). When samples with misplaced gels are encountered, whether during visual inspection by the laboratory staff or after it causes technical problems if undetected, the presence of such information would be time and money saving by avoiding resampling, additional testing, and communication efforts with the clinicians.