Introduction
A postmenopause, a physiological state in the woman life, is associated with the increased risk for cardiovascular disease (1-3). Risk factors include an atherogenic lipid profile, small increases of systolic and diastolic blood pressure and increased sympathetic tone. Furthermore, postmenopause is associated with central adiposity and increased insulin resistance (1,3). Among other, atherogenic lipid profile includes decreased or maintained level of high density lipoprotein (HDL) (1,3,4). Also, postmenopausal women show changes in the chemical composition of HDL, increased HDL oxidability and also decreased ability of HDL to prevent low density lipoprotein (LDL) oxidation (4).
One of the enzymes on HDL which possesses antiatherogenic and antioxidant properties is paraoxonase 1 (PON1) (5,6). PON1 is synthesized in the liver, secreted in the plasma where it is mainly associated with HDL (6,7). The N-terminal hydrophobic signal peptide of PON1 is structural requirement for bounding of the enzyme on the HDL (5). The association with HDL is necessary for obtaining the optimal enzyme stability and activity (8,9). Enzyme hydrolyses different substrates by its organophosphatase, arylesterase and lactonase activity (6,10-14). It was shown that PON1 has important physiological role in lipid metabolism and prevention of atherosclerosis. It protects HDL against oxidation and preserves its function, protects LDL against oxidation and decreases lipid peroxide in atherosclerotic lesions (6). The different non-genetic factors (like smoking, alcohol consumption, diets and different physiological and pathological states) and genetic factors [polymorphism of paraoxonase 1 (pon1) gene] affect PON1 activity which show large interindividual variability (up to 40 times) (5,6,15-18). PON1 phenotype is influenced by genetic and non-genetic factors (5,15).
Zago and co-workers reported changes in concentration, composition and function of HDL in postmenopausal women (4) which could have the effect on the PON1 activity. We assume that reduced activity of antioxidant enzyme like PON1 could also contribute to the higher risk of cardiovascular events in postmenopausal women. So, we aimed to investigate difference in the lipid status, paraoxonase and arylesterase PON1 activities and PON1 phenotype between women with regular menstrual cycle and postmenopausal women.
Materials and methods
Subjects
This case-control study was conducted in Department of medical biochemistry and haematology, Faculty of Pharmacy and Biochemistry, University of Zagreb and Polyclinic Bonifarm, Zagreb. Subjects were recruited during December 2013 in Polyclinic Aviva, Zagreb and Department of Gynaecology and Obstetrics, Medical School University Hospital Sestre Milosrdnice, Zagreb, Croatia.
The study included 23 apparently healthy women with spontaneous natural menopause and with normal cervical smear. Also, 51 apparently healthy premenopausal women with regular menstrual cycle and normal cervical smear were included in the study. 25 of them were in the follicular phase of the cycle (blood was collected from 3rd-12th day of cycle) and 26 were in luteal phase of the cycle (blood was collected from 19th day of cycle). All women were examined by general practitioner and gynaecologist. Health and menstrual status (pre- and postmenopausal) was defined by the questionnaire, and self-reported during interviews with the physicians. World Health Organization definition for postmenopausal was used, it consider postmenopausal status as absence of menstruation for at least 12 months (19). Exclusion criteria were: cardiovascular, renal, liver or neoplastic disease, diabetes mellitus and diseases of reproductive organs, use of oral contraceptive levonorgestrel-releasing intrauterine systemor hormone replacement therapy, pregnant women and women with surgical-evoked menopause.
The study was approved by the Ethic Committee of Medical School University Hospital Sestre Milosrdnice, Zagreb, Croatia and Polyclinic Aviva, Zagreb, Croatia. All participants signed informed consent.
Samples
Samples were collected after 12 hour of fasting, into serum tubes with clot activator (Greiner Bio-One, Kremsmünster, Austria). After 30 minutes of resting for spontaneous clotting, samples were centrifuged at 2000 x g for 10 minutes at room temperature and sera were stored at -20 °C until further analysis.
Methods
Concentrations of triglyceride, total cholesterol, HDL cholesterol, LDL cholesterol, apolipoprotein AI (apoAI) and apolipoprotein B (apoB) were determined on Cobas Integra 400 plus (Roche, Mannheim, Germany) with original reagents and according to manufacturer protocol (Roche, Mannheim, Germany).
PON1 activity in serum was assessed by using two different substrates: paraoxon (PON1 paraoxonase activity) and phenylacetate (PON1 arylesterase activity).
PON1 paraoxonase activity was measured in the absence and in the presence of NaCl (basal and salt-stimulated paraoxonase activity) on the BC-AU680 analyzer (Beckman Coulter, Brea, CA, USA) at 37 °C, as described previously (20). Briefly, reaction mixture contained 15 μL of serum and 300 μL of reagent [2.5 mmol/L paraoxon of ~ 90% purity (Sigma Aldrich Chemica GmbH, Steinheim, Germany) 2.2 mmol/L CaCl2(Kemika, Zagreb, Croatia) in 0.1 mol/L Tris-HCl buffer, pH 8.0 (Sigma Aldrich Chemica GmbH, Steinheim, Germany)]. For salt-stimulated paraoxonase activity buffer contained 1.0 mol/L NaCl. The release of p-nitrophenol was measured at 410/480 nm (ε = 17900 l/mol cm) and the PON1 enzyme activity was calculated. According to our data, intra-assay coefficients of variation (CV’s) were 1.42% and 1.75% for basal and NaCl stimulated PON1 activity, respectively. The quality of obtained results was monitoring by participating in interlaboratory comparisons.
Paraoxonase PON1 activity was presented as a basal (POX) and salt stimulated (POX1) paraoxonase activity and as activities standardized on the concentration of HDL (POX/HDL and POX1/HDL) and ApoA1 (POX/ApoA and POX1/ApoA).
PON1 arylesterase activity was determined by previously described method (20), which was modified for determination on microplate reader (1420 Victor3, PerkinElmer, Waltham, MA, USA). Stock solution of substrate [100 mmol/L phenylacetate, 99% purity (Sigma Aldrich Chemica GmbH, Steinheim, Germany) in 40% methanol (Kemika, Zagreb, Croatia)] was diluted 5x while serum samples were diluted 100x with 0.1 mol/L Tris-HCl buffer, pH 8.0 which contained 2 mmol/L CaCl2. Reaction mixture for determination of arylesterase activity in serum samples (total volume 0.3 mL) contained 0.24 mL buffer (0.1 mol/L Tris-HCl buffer, pH 8.0 which contained 2 mmol/L CaCl2), 0.03 mL 20 mmol/L phenylacetate and 0.03 mL of diluted serum. Reaction mixture for determination of spontaneous substrate hydrolysis (total volume 0.3 mL) contained 0.27 mL buffer (0.1 mol/L Tris-HCl buffer, pH 8.0 which contained 2 mmol/L CaCl2) and 0.03 mL 20 mmol/L phenylacetate. The release of phenol was measured at room temperature continuously (every 30 seconds) during 4 minutes at 260 nm (ε = 1310 l/mol cm) on the microplate reader. Calculated PON1 arylesterase activities in sera samples were corrected for spontaneous substrate hydrolysis.
Arylesterase PON1 activity (ARE) was also standardized on the concentration of HDL (ARE/HDL) and ApoA1 (ARE/ApoA1).
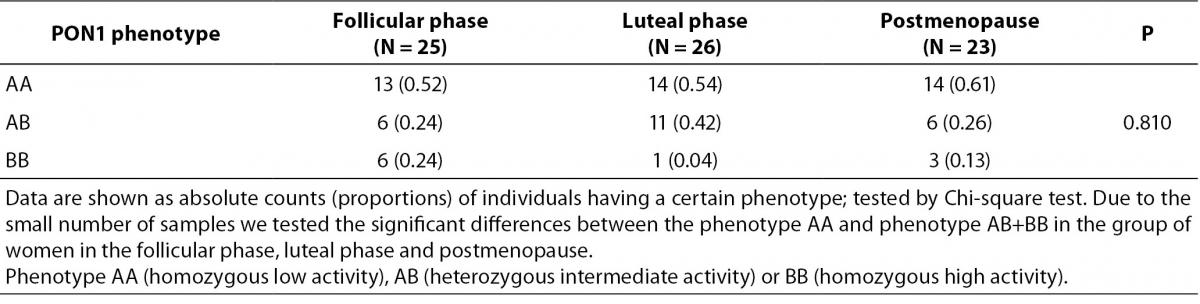
PON1 phenotype was determined by double substrate method (21). Briefly, phenotype is defined as the activity ratio of NaCl stimulated paraoxonase activity divided by arylesterase activity. Calculation was performed for each individual and was used for assigning subjects to one of the PON1 phenotypes AA (homozygous low activity), AB (heterozygous intermediate activity) or BB (homozygous high activity). The cumulative distribution of individuals with respect to arylesterase activity is unimodal and with respect to paraoxonase activity is bimodal. The cumulative distribution of the rate of paraoxonase to arylesterase activities is trimodal. This ratio divides the populations at the two antimodes (21). In our study two antimodes in group of women in follicular phase of menstrual cycle were 2.63 and 5.78 (AA phenotype activity ratio 2.63 and lower; BB phenotype activity ratio 5.78 and higher, AB phenotype activity ratio higher from 2.63 and lower from 5.78). Two antimodes in group of women in lutheal phase of menstrual cycle were 3.97 and 8.83 (AA phenotype activity ratio 3.97 and lower; BB phenotype activity ratio 8.83 and higher, AB phenotype activity ratio higher from 3.97 and lower from 8.83). Antimodes in group of postmenopausal women were 2.69 and 7.53 (AA phenotype activity ratio 2.69 and lower; BB phenotype activity ratio 7.53 and higher, AB phenotype activity ratio higher from 2.69 and lower from 7.53).
Statistical analysis
Statistical analysis was performed using SigmaStat for Windows, version 3.0 (2003. SPSS Inc, Erkrath, Germany). Data are presented as median (interquartile range) or as counts (proportions). Age is presented as median and range (minimum and maximum). Due to the small study group we used non-parametric statistic. Quantitative data were tested using the Kruskal-Wallis Analysis of Variance on Ranks and Dunn’s method was used for Post hoc testing. Chi-square test was used for comparison of proportions. P values lower than 0.05 were considered statistically significant.
Results
Median age of postmenopausal women subgroup (N = 23) was 55 (45-69) years. Group of premenopausal females (N = 51) were divided on two subgroups according to the menstrual cycle phase; follicular phase (N = 25) with median age of 36 (23-49) years and luteal phase (N = 26) with median age of 36 (24-46) years.
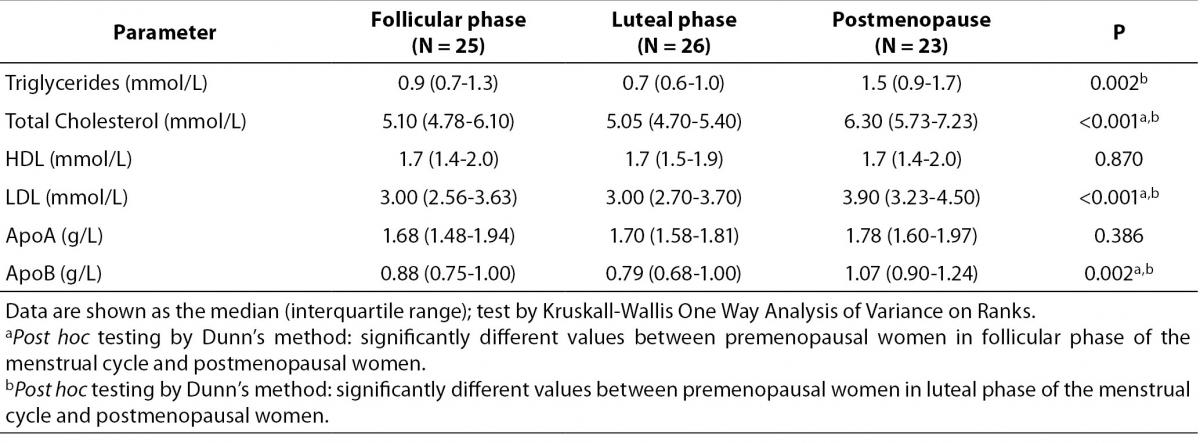
Lipid parameters for the study groups are presented in the Table 1. Compared to the both premenopausal groups, postmenopausal women had significantly higher concentration of total cholesterol, triglycerides, LDL cholesterol and ApoB. No differences in concentration of HDL cholesterol and ApoA1 were found between all groups. Post hoc testing showed significant difference in concentration of cholesterol, LDL cholesterol and ApoB between postmenopausal women and premenopausal women in follicular and in luteal phase of the menstrual cycle. On the other hand, post hoc test showed that concentration of triglyceride is different only between postmenopausal women and premenopausal women in the luteal phase of the cycle.
Table 1. Lipid parameters in premenopausal and postmenopausal women.
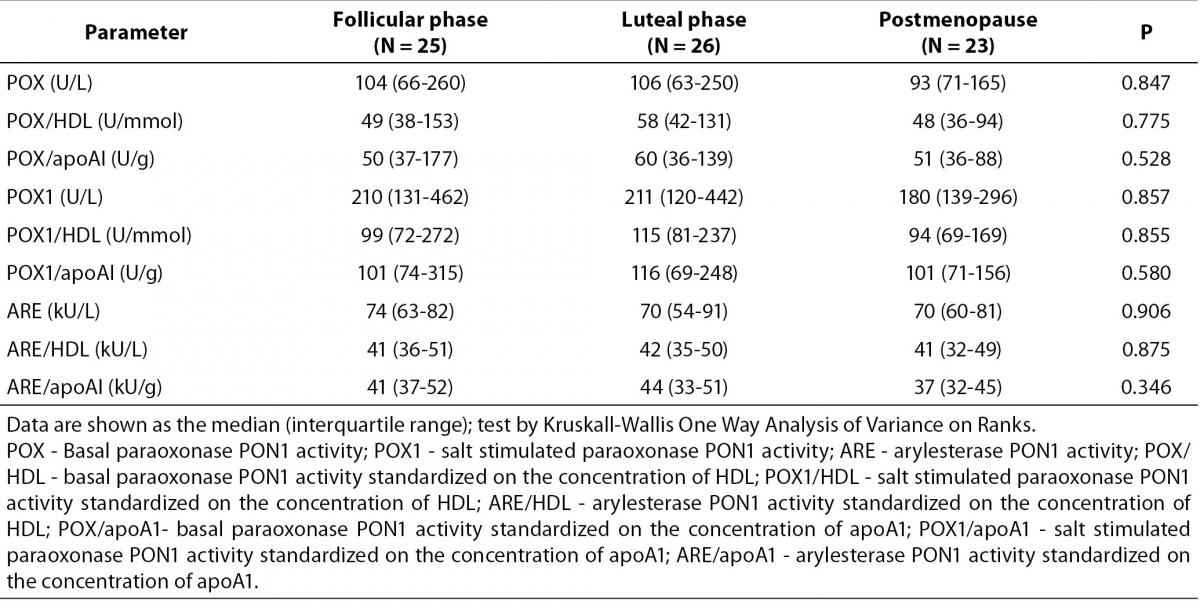
Table 2. shows paraoxonase and arylesterase PON1 activity and this activities standardized on the concentration of HDL and apoA1 in the tested groups. There were no significant differences in the POX, POX1 and ARE and in the standardized activities of PON1 between premenopausal and postmenopausal women. Table 3. shows the number and frequency of individuals having certain phenotype. Distribution of PON1 phenotypes was not different in the pre- and postmenopausal women.
Table 2. PON1 paraoxonase and arylesterase activities in premenopausal and postmenopausal women.
Table 3. PON1 phenotype in premenopausal and postmenopausal women.
Discussion
Our study was performed to investigate differences in the lipid profile, paraoxonase and arylesterase PON1 activity and PON1 phenotype in premenopausal and postmenopausal women. Our result show atherogenic lipid profile in postmenopausal women, higher concentrations of triglycerides, total cholesterol, LDL cholesterol and apoB. On the other hand paraoxonase and arylesterase PON1 activities as well as PON1 phenotype were similar in pre- and postmenopausal women.
Menopause is the physiological phase in the women’s life which is the result of the changes in their hormonal status (2). It was shown that higher cardiovascular risk in postmenopausal women is consequence of estrogen deficiency and ageing (2). Estrogen had indirect protective effects on lipid and glycaemic metabolism; furthermore it has direct effect on vessel function (2). Postmenopause is associated with pro-atherogenic lipid profile, central adiposity, increased diastolic pressure and increased insulin resistance (1). Pro-atherogenic lipid profile is characterized in general with higher concentration of total cholesterol, LDL cholesterol, triglyceride and lipoprotein (a) (Lpa) (1,3). Furthermore, HDL cholesterol is maintained or decreased in postmenopausal women (4).
In our study, we compared the lipid profile of the healthy premenopausal (follicular and lutheal phase) and postmenopausal women. As it was expected the postmenopausal women have pro-atherogenic lipid profile; higher concentration of total and LDL cholesterol and apoB, which is in accordance with previously published papers (4,22). Furthermore, we found higher concentration of triglycerides in postmenopausal women compared to the premenopausal women. Interestingly, only women in luteal phase of the cycle show significant differences. This could be result of the differences in the hormone status in these two phases of the menstrual cycle and this finding must be confirmed in further studies which would include large number of subjects. Other authors also reported significantly higher triglyceride concentration in postmenopausal women (4,22). Concentration of the HDL cholesterol and apoAI was similar in three examined group. The higher concentration of LDL cholesterol and unchanged concentration of HDL cholesterol could be risk factor for coronary heart disease. The results of lipid profile in postmenopausal women are not consistent. For example, Zago and co-workers reported higher concentration of triglycerides, total and LDL cholesterol and apoB, lower concentration of HDL and unchanged concentration of apoAI in postmenopausal women (4). Mascarenhas-Melo et al. showed significantly higher concentration of LDL, lower concentration of HDL and unchanged concentration of total cholesterol and triglyceride in postmenopausal women (23). On the other hand, Wakatsuki et al. did not find significant differences in the concentration of triglyceride, HDL cholesterol and ApoA1 between pre- and postmenopausal women and women with surgically caused menopause. They also reported significantly higher concentration of total and LDL cholesterol and ApoB in the examined groups (24). Interestingly, one paper did not find any significant differences in the lipid profile (cholesterol, triglycerides, LDL) between healthy pre- and postmenopausal women (25). The discrepancy in the results could be caused by differences in the study design, different ethnicity and lifestyles habits.
Furthermore, in this paper we determined the enzyme activity and phenotype of PON1 enzyme. It is well know that PON1 have antiatherogenic properties. It protects HDL and LDL from oxidation and destroys biologically active oxidized lipids on lipoproteins and arterial cells (6,26,27). The association of PON1 with HDL is important for serum enzyme activity. HDL stimulates secretion and stabilizes the secreted enzyme (9). Apo AI is not necessary for binding of PON1 on HDL but is important for enzyme stability and activity (5,8,9). PON1 is not associated with all types of HDL partials. Less than 10% of total HDL reacts with anti-PON1 antibodies (9). Changes in HDL concentration, HDL composition and in distribution of HDL subfraction could lead to the change in PON1 activity. Zago and co-authors showed that postmenopausal women have changed HDL chemical composition (4). HDL from postmenopausal women has higher content of triglycerides and lower concentration of cholesterol compared to the premenopausal women (4). Only few papers examined the PON1 activity in postmenopausal women and paraoxon was usually used as the substrate. The paraoxonase activity of PON1 gives the information of the hydrolytic activity of the enzyme toward substrate paraoxon. Capacity of PON1 to protect LDL from oxidation is reversed to its paraoxon activity so the measurement of PON1 paraoxonase activity gives very limited information (28). On the other hand, arylesterase activity could be used for the estimation of PON1 concentration in serum (5,29). In this study, we determined both paraoxonase and arylesterase activity in all examined group, and did not detect significant differences in either of PON1 activities. Furthermore, we standardized paraoxonase and arylesterase activities of PON1 to the concentration of HDL and apoA1. Standardized activity was also similar in all three groups examined as well as distribution of PON1 phenotype. Results regarding PON1 activity, which are reported in the literature, are also inconsistent. Zago reported that pre- and postmenopausal women had similar basal and salt stimulated paraoxonase PON1 activity as well as arylesterase PON1 activity (4). The absence of differences in paraoxonase PON1 activity was also reported in two papers (23,25). However, Topçuoglu and co-workers reported significantly lower paraoxonase PON1 activity in postmenopausal women (22). It is well know that different non-genetic and genetic factors effect PON1 activity (6,15-18). Discrepancies in the results obtained could be consequence of different genotype distribution of polymorphisms in promoter or coding region of the pon1 gene which influence concentration, or activity of the enzyme. Furthermore different lifestyle habits could also explain different results.
In this paper we did not show significant difference in the PON1 enzyme activities in postmenopausal women, as compared to the women in follicular and luteal phase of the menstrual cycle. In contrast to most of the authors we used two substrates for determination of the PON1 activity and we also standardized these activities to concentration of HDL and apoA1. We confirmed findings of other authors that postmenopausal women have pro-atherogenic lipid profile. However, concentration of HDL was not changed in postmenopausal women. The results demonstrated that postmenopausal women had a disturbance in the lipid profile and increased risk of atherogenesis. However, the results did not establish a connection between PON1 activity and risk of atherosclerosis. The limitation of our study was small number of studied subjects, but other authors have also used group of 50 or less study subjects (4,22,23,25). In order to obtain more reliable results further studies must include larger number of subjects. Further studies are needed to examine PON1 antioxidant ability in preventing oxidation of LDL and HDL in postmenopausal women.
In conclusion, the results of our study showed that there are no differences in PON1 activity and PON1 phenotype between women with regular menstrual cycle and postmenopausal women.
Acknowledgements
This work was supported by the Croatian Ministry of Science, Education and Sports (grant number 006-0061245-0977).